-
 Deciphering Plant-Insect-Microorganism Signals for Sustainable Crop Production
Deciphering Plant-Insect-Microorganism Signals for Sustainable Crop Production -
 Leptin: A Heavyweight Player in Obesity-Related Cancers
Leptin: A Heavyweight Player in Obesity-Related Cancers -
 Nuclear Phosphoinositides as Key Determinants of Nuclear Functions
Nuclear Phosphoinositides as Key Determinants of Nuclear Functions -
 The Outstanding Chemodiversity of Marine-Derived Talaromyces
The Outstanding Chemodiversity of Marine-Derived Talaromyces -
 Mitigating Oxidative Stress in Perinatal Cells: A Critical Step toward an Optimal Therapeutic Use in Regenerative Medicine
Mitigating Oxidative Stress in Perinatal Cells: A Critical Step toward an Optimal Therapeutic Use in Regenerative Medicine
Journal Description
Biomolecules
Biomolecules
is a peer-reviewed, open access journal on structures and functions of bioactive and biogenic substances, molecular mechanisms with biological and medical implications as well as biomaterials and their applications. Biomolecules is published monthly online by MDPI. The Spanish Society for Biochemistry and Molecular Biology (SEBBM) is affiliated with Biomolecules and their members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, MEDLINE, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Biochemistry & Molecular Biology) / CiteScore - Q1 (Biochemistry)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.2 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the first half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Sections: published in 19 topical sections.
- Testimonials: See what our editors and authors say about Biomolecules.
- Companion journal: Receptors.
Impact Factor:
5.5 (2022);
5-Year Impact Factor:
5.8 (2022)
Latest Articles
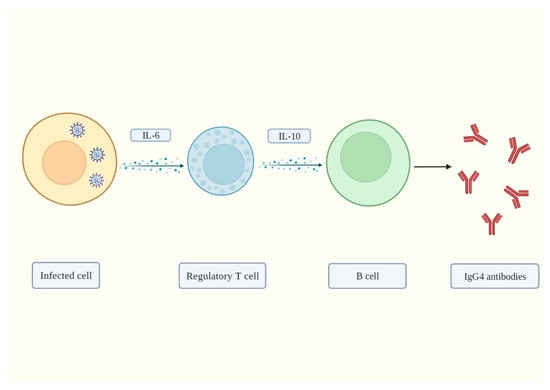
Does SARS-CoV-2 Induce IgG4 Synthesis to Evade the Immune System?
Biomolecules 2023, 13(9), 1338; https://doi.org/10.3390/biom13091338 (registering DOI) - 01 Sep 2023
Abstract
►
Show Figures
SARS-CoV-2, the virus that causes the COVID-19 disease, has been shown to cause immune suppression in certain individuals. This can manifest as a reduced ability of the host’s immune system to effectively control the infection. Studies have reported that patients with COVID-19 can
[...] Read more.
SARS-CoV-2, the virus that causes the COVID-19 disease, has been shown to cause immune suppression in certain individuals. This can manifest as a reduced ability of the host’s immune system to effectively control the infection. Studies have reported that patients with COVID-19 can exhibit a decline in white blood cell counts, including natural killer cells and T cells, which are integral components of the immune system’s response to viral pathogens. These cells play critical roles in the immune response to viral infections, and their depletion can make it harder for the body to mount an effective defense against the virus. Additionally, the virus can also directly infect immune cells, further compromising their ability to function. Some individuals with severe COVID-19 pneumonia may develop a “cytokine storm”, an overactive immune response that may result in tissue damage and organ malfunction. The underlying mechanisms of immune suppression in SARS-CoV-2 are not entirely understood at this time, and research is being conducted to gain a more comprehensive understanding. Research has shown that severe SARS-CoV-2 infection promotes the synthesis of IgG4 antibodies. In this study, we propose the hypothesis that IgG4 antibodies produced by B cells in response to infection by SARS-CoV-2 generate immunological tolerance, which prevents its elimination and leads to persistent and chronic infection. In summary, we believe that this constitutes another immune evasion mechanism that bears striking similarities to that developed by cancer cells to evade immune surveillance.
Full article
Open AccessArticle
Characterization of the Abracl-Expressing Cell Populations in the Embryonic Mammalian Telencephalon
by
, , , , , and
Biomolecules 2023, 13(9), 1337; https://doi.org/10.3390/biom13091337 - 31 Aug 2023
Abstract
Abracl (ABRA C-terminal-like protein) is a small, non-typical winged-helix protein that shares similarity with the C-terminal domain of the protein ABRA (Actin-Binding Rho-Activating protein). The role of Abracl in the cell remains elusive, although in cancer cells, it has been implicated in proliferation,
[...] Read more.
Abracl (ABRA C-terminal-like protein) is a small, non-typical winged-helix protein that shares similarity with the C-terminal domain of the protein ABRA (Actin-Binding Rho-Activating protein). The role of Abracl in the cell remains elusive, although in cancer cells, it has been implicated in proliferation, migration and actin dynamics. Our previous study showed that Abracl mRNA was expressed in the dividing cells of the subpallial subventricular zone (SVZ), in the developing cortical plate (CP), and in the diencephalic SVZ; however, the molecular identities of the Abracl-expressing cell populations were not defined in that work. In this study, we use double immunofluorescence to characterize the expression of Abracl on sections of embryonic murine (E11.5-E18.5) and feline (E30/31-E33/34) telencephalon; to this end, we use a battery of well-known molecular markers of cycling (Ki67, Ascl1, Dlx2) or post-mitotic (Tubb3, Gad65/67, Lhx6 and Tbr1) cells. Our experiments show that Abracl protein has, compared to the mRNA, a broader expression domain, including, apart from proliferating cells of the subpallial and diencephalic SVZ, post-mitotic cells occupying the subpallial and pallial mantle (including the CP), as well as subpallial-derived migrating interneurons. Interestingly, in late embryonic developmental stages, Abracl was also transiently detected in major telencephalic fiber tracts.
Full article
(This article belongs to the Special Issue Neuroanatomy as an Implement for the Study of CNS Function and Dysfunction)
►▼
Show Figures
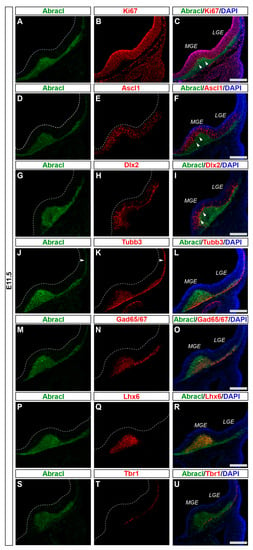
Figure 1
Open AccessArticle
Olfactory Dysfunction Is Associated with Cerebral Amyloid Deposition and Cognitive Function in the Trajectory of Alzheimer’s Disease
Biomolecules 2023, 13(9), 1336; https://doi.org/10.3390/biom13091336 - 31 Aug 2023
Abstract
Olfactory dysfunction is consistently observed in individuals with Alzheimer’s disease (AD), but its association with beta-amyloid (Aβ) deposition remains unclear. This study aimed to investigate the relationship among olfactory function, cerebral Aβ deposition, and neuropsychological profiles in individuals with no cognitive impairment (NCI),
[...] Read more.
Olfactory dysfunction is consistently observed in individuals with Alzheimer’s disease (AD), but its association with beta-amyloid (Aβ) deposition remains unclear. This study aimed to investigate the relationship among olfactory function, cerebral Aβ deposition, and neuropsychological profiles in individuals with no cognitive impairment (NCI), mild cognitive impairment (MCI), and AD dementia. A total of 164 participants were included, and olfactory function was assessed using the brief smell identification test (B-SIT). Cerebral Aβ deposition was measured using [18F]-flutemetamol PET imaging (A-PET). The results show a significant group difference in olfactory function, with the highest impairment observed in the Aβ-positive MCI and AD dementia groups, and the impairment was the lowest in Aβ-negative NCI. Olfactory dysfunction was positively associated with cognitive impairments across multiple domains. Furthermore, individuals with Aβ deposition had lower olfactory function compared to those without Aβ, even within the same neuropsychological stage. The association between olfactory dysfunction and Aβ deposition was observed globally and in specific cortical regions. These findings suggest that olfactory dysfunction is associated with both cognitive function and cerebral Aβ pathology in the trajectory of AD. Olfactory deficits may serve as an additional marker for disease progression and contribute to understanding the underlying mechanisms of AD.
Full article
(This article belongs to the Special Issue Advances in Biomarkers for Neurodegenerative Diseases)
►▼
Show Figures
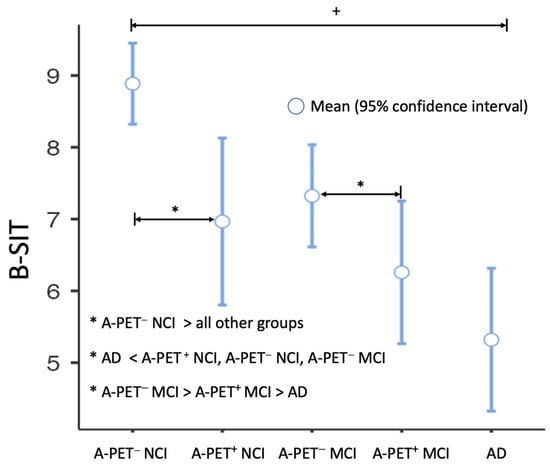
Figure 1
Open AccessArticle
Cisplatin Dependent Secretion of Immunomodulatory High Mobility Group Box 1 (HMGB1) Protein from Lung Cancer Cells
Biomolecules 2023, 13(9), 1335; https://doi.org/10.3390/biom13091335 - 31 Aug 2023
Abstract
High mobility group box 1 (HMGB1) is secreted from activated immune cells, necrotic cells, and certain cancers. Previous studies have reported that different patterns of post-translational modification, particularly acetylation and oxidation, mediate HMGB1 release and confer distinct extracellular HMGB1 signaling activity. Here we
[...] Read more.
High mobility group box 1 (HMGB1) is secreted from activated immune cells, necrotic cells, and certain cancers. Previous studies have reported that different patterns of post-translational modification, particularly acetylation and oxidation, mediate HMGB1 release and confer distinct extracellular HMGB1 signaling activity. Here we report that cisplatin but not carboplatin induces secretion of HMGB1 from human A549 non-small cell lung cancer (NSCLC) cells. Cisplatin-mediated HMGB1 secretion was dose-dependent and was regulated by nuclear exportin 1 (XPO1) also known as chromosomal maintenance 1 (CRM1) rather than adenosine diphosphate (ADP)-ribosylation, acetylation, or oxidation. HMGB1, as well as lysine acetylation and cysteine disulfide oxidation of secreted HMGB1, were monitored by sensitive and specific assays using immunoprecipitation, stable isotope dilution, differential alkylation, and nano liquid chromatography parallel reaction monitoring/high-resolution mass spectrometry (nano-LC-PRM/HRMS). A major fraction of the HMGB1 secreted by low-dose cisplatin treatment of A549 NSCLC cells was found to be in the fully reduced form. In contrast, mainly oxidized forms of HMGB1 were secreted by dimethyl sulfoxide (DMSO)-mediated apoptosis. These findings suggest that inhibition of XPO1 could potentiate the anti-tumor activity of cisplatin by increasing the nuclear accumulation of HMGB1 protein, an inhibitor of cisplatin DNA-adduct repair. Furthermore, low-dose cisplatin therapy could modulate the immune response in NSCLC through the established chemokine activity of extracellular reduced HMGB1. This could potentially enhance the efficacy of subsequent immunotherapy treatment.
Full article
(This article belongs to the Topic Chromatography–Mass Spectrometry Analysis in Biomedical Research and Clinical Laboratory)
►▼
Show Figures
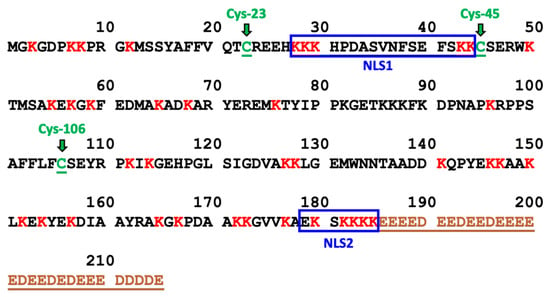
Figure 1
Open AccessArticle
Extracellular Acetylated Histone 3.3 Induces Inflammation and Lung Tissue Damage
by
, , , , , , , and
Biomolecules 2023, 13(9), 1334; https://doi.org/10.3390/biom13091334 - 31 Aug 2023
Abstract
Extracellular histones, part of the protein group known as damage-associated molecular patterns (DAMPs), are released from damaged or dying cells and can instigate cellular toxicity. Within the context of chronic obstructive pulmonary disease (COPD), there is an observed abundance of extracellular histone H3.3,
[...] Read more.
Extracellular histones, part of the protein group known as damage-associated molecular patterns (DAMPs), are released from damaged or dying cells and can instigate cellular toxicity. Within the context of chronic obstructive pulmonary disease (COPD), there is an observed abundance of extracellular histone H3.3, indicating potential pathogenic implications. Notably, histone H3.3 is often found hyperacetylated (AcH3.3) in the lungs of COPD patients. Despite these observations, the specific role of these acetylated histones in inducing pulmonary tissue damage in COPD remains unclear. To investigate AcH3.3’s impact on lung tissue, we administered recombinant histones (rH2A, rH3.3, and rAcH3.3) or vehicle solution to mice via intratracheal instillation. After 48 h, we evaluated the lung toxicity damage and found that the rAcH3.3 treated animals exhibited more severe lung tissue damage compared to those treated with non-acetylated H3.3 and controls. The rAcH3.3 instillation resulted in significant histological changes, including alveolar wall rupture, epithelial cell damage, and immune cell infiltration. Micro-CT analysis confirmed macroscopic structural changes. The rAcH3.3 instillation also increased apoptotic activity (cleavage of caspase 3 and 9) and triggered acute systemic inflammatory marker activation (TNF-α, IL-6, MCP-3, or CXCL-1) in plasma, accompanied by leukocytosis and lymphocytosis. Confocal imaging analysis confirmed lymphocytic and monocytic/macrophage lung infiltration in response to H3.3 and AcH3.3 administration. Taken together, our findings implicate extracellular AcH3.3 in inducing cytotoxicity and acute inflammatory responses, suggesting its potential role in promoting COPD-related lung damage progression.
Full article
(This article belongs to the Special Issue Molecular Research on Airway Diseases)
►▼
Show Figures
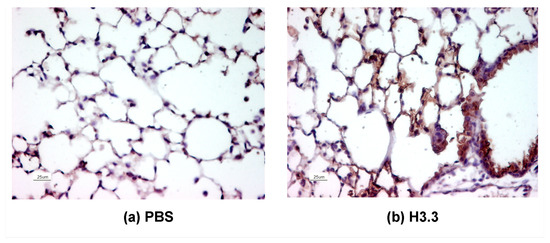
Figure 1
Open AccessArticle
ABCD1 Transporter Deficiency Results in Altered Cholesterol Homeostasis
by
, , , , , , , , and
Biomolecules 2023, 13(9), 1333; https://doi.org/10.3390/biom13091333 - 31 Aug 2023
Abstract
X-linked adrenoleukodystrophy (X-ALD), the most common peroxisomal disorder, is caused by mutations in the peroxisomal transporter ABCD1, resulting in the accumulation of very long-chain fatty acids (VLCFA). Strongly affected cell types, such as oligodendrocytes, adrenocortical cells and macrophages, exhibit high cholesterol turnover. Here,
[...] Read more.
X-linked adrenoleukodystrophy (X-ALD), the most common peroxisomal disorder, is caused by mutations in the peroxisomal transporter ABCD1, resulting in the accumulation of very long-chain fatty acids (VLCFA). Strongly affected cell types, such as oligodendrocytes, adrenocortical cells and macrophages, exhibit high cholesterol turnover. Here, we investigated how ABCD1 deficiency affects cholesterol metabolism in human X-ALD patient-derived fibroblasts and CNS tissues of Abcd1-deficient mice. Lipidome analyses revealed increased levels of cholesterol esters (CE), containing both saturated VLCFA and mono/polyunsaturated (V)LCFA. The elevated CE(26:0) and CE(26:1) levels remained unchanged in LXR agonist-treated Abcd1 KO mice despite reduced total C26:0. Under high-cholesterol loading, gene expression of SOAT1, converting cholesterol to CE and lipid droplet formation were increased in human X-ALD fibroblasts versus healthy control fibroblasts. However, the expression of NCEH1, catalysing CE hydrolysis and the cholesterol transporter ABCA1 and cholesterol efflux were also upregulated. Elevated Soat1 and Abca1 expression and lipid droplet content were confirmed in the spinal cord of X-ALD mice, where expression of the CNS cholesterol transporter Apoe was also elevated. The extent of peroxisome-lipid droplet co-localisation appeared low and was not impaired by ABCD1-deficiency in cholesterol-loaded primary fibroblasts. Finally, addressing steroidogenesis, progesterone-induced cortisol release was amplified in X-ALD fibroblasts. These results link VLCFA to cholesterol homeostasis and justify further consideration of therapeutic approaches towards reducing VLCFA and cholesterol levels in X-ALD.
Full article
(This article belongs to the Special Issue The Role of Peroxisome in Cell Functions and Pathological Consequences of Its Dysfunction)
►▼
Show Figures
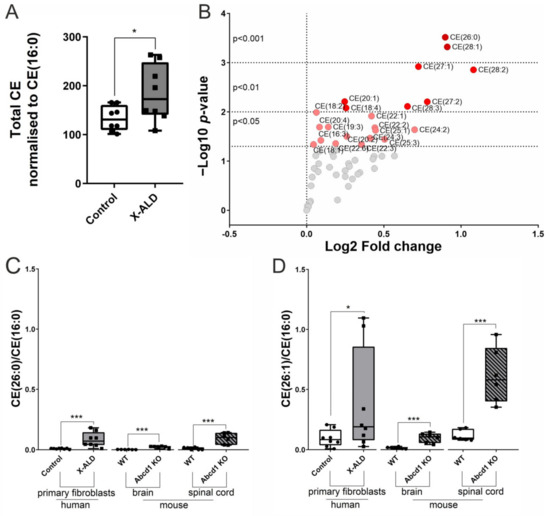
Figure 1
Open AccessReview
Infection, Inflammation, and Immunity in Sepsis
Biomolecules 2023, 13(9), 1332; https://doi.org/10.3390/biom13091332 - 31 Aug 2023
Abstract
Sepsis is triggered by microbial infection, injury, or even major surgery. Both innate and adaptive immune systems are involved in its pathogenesis. Cytoplasmic presence of DNA or RNA of the invading organisms or damaged nuclear material (in the form of micronucleus in the
[...] Read more.
Sepsis is triggered by microbial infection, injury, or even major surgery. Both innate and adaptive immune systems are involved in its pathogenesis. Cytoplasmic presence of DNA or RNA of the invading organisms or damaged nuclear material (in the form of micronucleus in the cytoplasm) in the host cell need to be eliminated by various nucleases; failure to do so leads to the triggering of inflammation by the cellular cGAS-STING system, which induces the release of IL-6, TNF-α, and IFNs. These cytokines activate phospholipase A2 (PLA2), leading to the release of polyunsaturated fatty acids (PUFAs), gamma-linolenic acid (GLA), arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which form precursors to various pro- and anti-inflammatory eicosanoids. On the other hand, corticosteroids inhibit PLA2 activity and, thus, suppress the release of GLA, AA, EPA, and DHA. PUFAs and their metabolites have a negative regulatory action on the cGAS-STING pathway and, thus, suppress the inflammatory process and initiate inflammation resolution. Pro-inflammatory cytokines and corticosteroids (corticosteroids > IL-6, TNF-α) suppress desaturases, which results in decreased formation of GLA, AA, and other PUFAs from the dietary essential fatty acids (EFAs). A deficiency of GLA, AA, EPA, and DHA results in decreased production of anti-inflammatory eicosanoids and failure to suppress the cGAS-STING system. This results in the continuation of the inflammatory process. Thus, altered concentrations of PUFAs and their metabolites, and failure to suppress the cGAS-STING system at an appropriate time, leads to the onset of sepsis. Similar abnormalities are also seen in radiation-induced inflammation. These results imply that timely administration of GLA, AA, EPA, and DHA, in combination with corticosteroids and anti-IL-6 and anti-TNF-α antibodies, may be of benefit in mitigating radiation-induced damage and sepsis.
Full article
(This article belongs to the Collection Feature Papers in Biomacromolecules: Lipids)
►▼
Show Figures
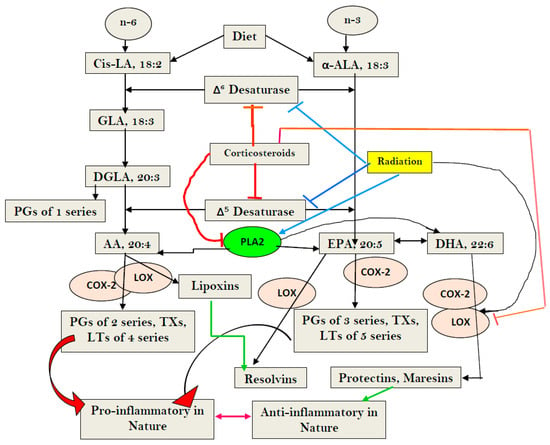
Figure 1
Open AccessArticle
FOXM1a Isoform of Oncogene FOXM1 Is a Tumor Suppressor Suppressed by hnRNP C in Oral Squamous Cell Carcinoma
Biomolecules 2023, 13(9), 1331; https://doi.org/10.3390/biom13091331 - 30 Aug 2023
Abstract
FOXM1 is an oncogenic transcriptional factor and includes several isoforms generated by alternative splicing. Inclusion of alternative exon 9 produces FOXM1a, a transcriptionally inactive isoform. However, the role of FOXM1a in tumorigenesis remains unknown. In addition, the regulatory mechanisms of exon 9 splicing
[...] Read more.
FOXM1 is an oncogenic transcriptional factor and includes several isoforms generated by alternative splicing. Inclusion of alternative exon 9 produces FOXM1a, a transcriptionally inactive isoform. However, the role of FOXM1a in tumorigenesis remains unknown. In addition, the regulatory mechanisms of exon 9 splicing are also unclear. In the present study, we found that overexpression of FOXM1a significantly reduced cell proliferation and colony formation of oral squamous cell carcinoma (OSCC) cell proliferation in vitro. Importantly, OSCC cells with FOXM1a overexpression showed significantly slower tumor formation in nude mice. Moreover, we identified a U-rich exonic splicing suppressor (ESS) which is responsible for exon 9 skipping. Splicing factor heterogeneous nuclear ribonucleoprotein C (hnRNP C) can bind to the ESS and suppress exon 9 inclusion and FOXM1a expression. Silence of hnRNP C also significantly suppresses OSCC cell proliferation. HnRNP C is significantly co-expressed with FOXM1 in cancers. Our study uncovered a novel regulatory mechanism of oncogene FOXM1 expression in OSCC.
Full article
(This article belongs to the Section Molecular Biology)
►▼
Show Figures
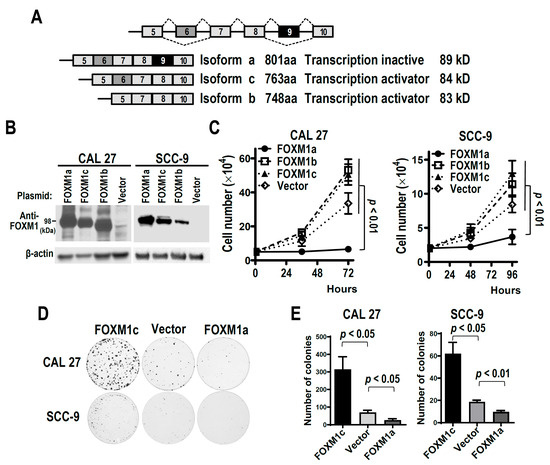
Figure 1
Open AccessArticle
Exploring the Therapeutic Potential of Ethyl 3-Hydroxybutyrate in Alleviating Skeletal Muscle Wasting in Cancer Cachexia
Biomolecules 2023, 13(9), 1330; https://doi.org/10.3390/biom13091330 - 30 Aug 2023
Abstract
Cachexia (CAC) is a debilitating metabolic syndrome. Although dietary interventions are attractive, long-term adherence to specific diets is difficult to maintain and can lead to systemic side effects. Ethyl 3-hydroxybutyrate (EHB) is a commonly used food additive found in wine and Tribolium castaneum.
[...] Read more.
Cachexia (CAC) is a debilitating metabolic syndrome. Although dietary interventions are attractive, long-term adherence to specific diets is difficult to maintain and can lead to systemic side effects. Ethyl 3-hydroxybutyrate (EHB) is a commonly used food additive found in wine and Tribolium castaneum. In this study, we investigated the effects of EHB administration in cachectic mice. After a single intraperitoneal injection of EHB into mice, 3-hydroxybutyrate (3-HB) levels were significantly increased in the serum and gastrocnemius of mice. The administration of EHB alleviated cachexia-related symptoms, ameliorated skeletal muscle atrophy, and improved survival in cachectic mice. In addition, the supplementation of cachectic mice with 3-HB by EHB administration significantly reduced tumor weights, indicating the anti-tumor effects of 3-HB. Remarkably, the addition of 3-HB to the culture medium significantly attenuated the C2C12 myotube atrophy induced by the culture supernatant of CT26 cell lines, highlighting its potential to counteract the destructive effects of tumor-derived elements on muscle tissue. NMR-based metabolomics analysis provided insights into the underlying mechanisms and revealed that the anti-cachexia effects of 3-HB treatment can be attributed to three key mechanisms: the promotion of the TCA cycle and the attenuation of proteolysis, the promotion of protein synthesis and the improvement of metabolic homeostasis, and a reduction in inflammation and an enhancement of the antioxidant capacity. This study provided compelling evidence for the protective effects of 3-HB treatment on the cachectic gastrocnemius and highlighted the efficacy of EHB administration as a ketone supplementation approach to achieve nutritional ketosis without the need for dietary restriction.
Full article
(This article belongs to the Section Molecular Biology)
►▼
Show Figures
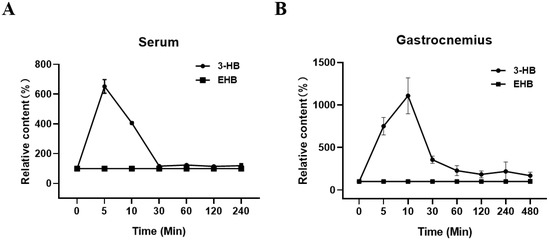
Figure 1
Open AccessArticle
Multidimensional Landscape of SA-AKI Revealed by Integrated Proteomics and Metabolomics Analysis
Biomolecules 2023, 13(9), 1329; https://doi.org/10.3390/biom13091329 - 30 Aug 2023
Abstract
Sepsis-associated acute kidney injury (SA-AKI) is a severe and life-threatening condition with high morbidity and mortality among emergency patients, and it poses a significant risk of chronic renal failure. Clinical treatments for SA-AKI remain reactive and non-specific, lacking effective diagnostic biomarkers or treatment
[...] Read more.
Sepsis-associated acute kidney injury (SA-AKI) is a severe and life-threatening condition with high morbidity and mortality among emergency patients, and it poses a significant risk of chronic renal failure. Clinical treatments for SA-AKI remain reactive and non-specific, lacking effective diagnostic biomarkers or treatment targets. In this study, we established an SA-AKI mouse model using lipopolysaccharide (LPS) and performed proteomics and metabolomics analyses. A variety of bioinformatic analyses, including gene set enrichment analysis (GSEA), weighted gene co-expression network analysis (WGCNA), protein and protein interactions (PPI), and MetaboAnalyst analysis, were conducted to investigate the key molecules of SA-AKI. Integrated proteomics and metabolomics analysis revealed that sepsis led to impaired renal mitochondrial function and metabolic disorders. Immune-related pathways were found to be activated in kidneys upon septic infection. The catabolic products of polyamines accumulated in septic kidneys. Overall, our integrated analysis provides a multidimensional understanding of SA-AKI and identifies potential pathways for this condition.
Full article
(This article belongs to the Special Issue Proteomics and Metabolomics Biomarkers in Different Diseases)
►▼
Show Figures
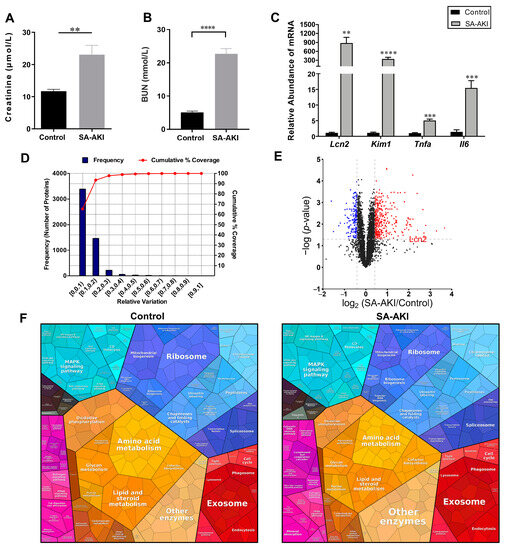
Figure 1
Open AccessArticle
Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia
by
, , , , , , and
Biomolecules 2023, 13(9), 1328; https://doi.org/10.3390/biom13091328 - 29 Aug 2023
Abstract
Inflammatory, vasculogenic, and profibrogenic factors have been previously reported in vitreous (VH) and aqueous (AH) humors in myopic patients who underwent cataract surgery. In light of this, we selected some mediators for AH and anterior-capsule-bearing lens epithelial cell (AC/LEC) analysis, and AH expression
[...] Read more.
Inflammatory, vasculogenic, and profibrogenic factors have been previously reported in vitreous (VH) and aqueous (AH) humors in myopic patients who underwent cataract surgery. In light of this, we selected some mediators for AH and anterior-capsule-bearing lens epithelial cell (AC/LEC) analysis, and AH expression was correlated with LEC activation (epithelial–mesenchymal transition and EMT differentiation) and axial length (AL) elongation. In this study, AH (97; 41M/56F) and AC/LEC samples (78; 35M/43F) were collected from 102 patients who underwent surgery, and biosamples were grouped according to AL elongation. Biomolecular analyses were carried out for AH and LECs, while microscopical analyses were restricted to whole flattened AC/LECs. The results showed increased levels of interleukin (IL)-6, IL-8, and angiopoietin-2 (ANG)-2 and decreased levels of vascular endothelium growth factor (VEGF)-A were detected in AH depending on AL elongation. LECs showed EMT differentiation as confirmed by the expression of smooth muscle actin (α-SMA) and transforming growth factor (TGF)-βR1/TGFβ isoforms. A differential expression of IL-6R/IL-6, IL-8R/IL-8, and VEGF-R1/VEGF was observed in the LECs, and this expression correlated with AL elongation. The higher VEGF-A and lower VEGF-D transcript expressions were detected in highly myopic LECs, while no significant changes were monitored for VEGF-R transcripts. In conclusion, these findings provide a strong link between the AH protein signature and the EMT phenotype. Furthermore, the low VEGF-A/ANG-2 and the high VEGF-A/VEGF-D ratios in myopic AH might suggest a specific inflammatory and profibrogenic pattern in high myopia. The highly myopic AH profile might be a potential candidate for rating anterior chamber inflammation and predicting retinal distress at the time of cataract surgery.
Full article
(This article belongs to the Special Issue New Insights into the Molecular Mechanisms of Myopia and Glaucoma)
►▼
Show Figures
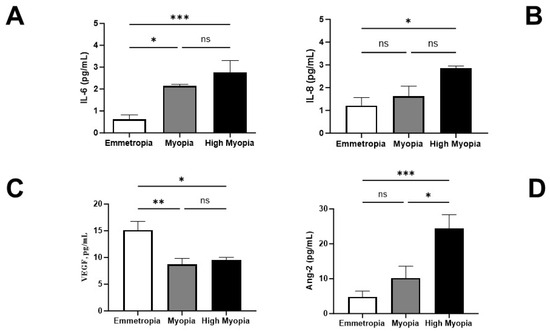
Figure 1
Open AccessReview
Artificial Intelligence Assists in the Detection of Blood Vessels in Whole Slide Images: Practical Benefits for Oncological Pathology
by
, , , , , and
Biomolecules 2023, 13(9), 1327; https://doi.org/10.3390/biom13091327 - 29 Aug 2023
Abstract
The analysis of the microvasculature and the assessment of angiogenesis have significant prognostic value in various diseases, including cancer. The search for invasion into the blood and lymphatic vessels and the assessment of angiogenesis are important aspects of oncological diagnosis. These features determine
[...] Read more.
The analysis of the microvasculature and the assessment of angiogenesis have significant prognostic value in various diseases, including cancer. The search for invasion into the blood and lymphatic vessels and the assessment of angiogenesis are important aspects of oncological diagnosis. These features determine the prognosis and aggressiveness of the tumor. Traditional manual evaluation methods are time consuming and subject to inter-observer variability. Blood vessel detection is a perfect task for artificial intelligence, which is capable of rapid analyzing thousands of tissue structures in whole slide images. The development of computer vision solutions requires the segmentation of tissue regions, the extraction of features and the training of machine learning models. In this review, we focus on the methodologies employed by researchers to identify blood vessels and vascular invasion across a range of tumor localizations, including breast, lung, colon, brain, renal, pancreatic, gastric and oral cavity cancers. Contemporary models herald a new era of computational pathology in morphological diagnostics.
Full article
(This article belongs to the Special Issue Digital Pathology 2.0)
►▼
Show Figures
Figure 1
Open AccessReview
Structure, Function, and Allosteric Regulation of the 20S Proteasome by the 11S/PA28 Family of Proteasome Activators
Biomolecules 2023, 13(9), 1326; https://doi.org/10.3390/biom13091326 - 29 Aug 2023
Abstract
The proteasome, a complex multi-catalytic protease machinery, orchestrates the protein degradation essential for maintaining cellular homeostasis, and its dysregulation also underlies many different types of diseases. Its function is regulated by many different mechanisms that encompass various factors such as proteasome activators (PAs),
[...] Read more.
The proteasome, a complex multi-catalytic protease machinery, orchestrates the protein degradation essential for maintaining cellular homeostasis, and its dysregulation also underlies many different types of diseases. Its function is regulated by many different mechanisms that encompass various factors such as proteasome activators (PAs), adaptor proteins, and post-translational modifications. This review highlights the unique characteristics of proteasomal regulation through the lens of a distinct family of regulators, the 11S, REGs, or PA26/PA28. This ATP-independent family, spanning from amoebas to mammals, exhibits a common architectural structure; yet, their cellular biology and criteria for protein degradation remain mostly elusive. We delve into their evolution and cellular biology, and contrast their structure and function comprehensively, emphasizing the unanswered questions regarding their regulatory mechanisms and broader roles in proteostasis. A deeper understanding of these processes will illuminate the roles of this regulatory family in biology and disease, thus contributing to the advancement of therapeutic strategies.
Full article
(This article belongs to the Special Issue Allosteric Regulation in Ubiquitin Proteasome System)
►▼
Show Figures
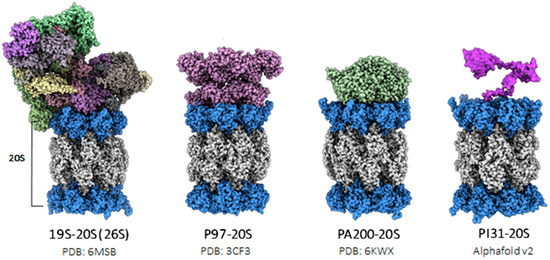
Figure 1
Open AccessArticle
Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy
by
, , , , and
Biomolecules 2023, 13(9), 1325; https://doi.org/10.3390/biom13091325 - 29 Aug 2023
Abstract
The pathological consequences of type 2 diabetes mellitus (T2DM) also involve the central nervous system; indeed, T2DM patients suffer from learning and memory disabilities with a higher risk of developing dementia. Although several factors have been proposed as possible contributors, how neuroactive steroids
[...] Read more.
The pathological consequences of type 2 diabetes mellitus (T2DM) also involve the central nervous system; indeed, T2DM patients suffer from learning and memory disabilities with a higher risk of developing dementia. Although several factors have been proposed as possible contributors, how neuroactive steroids and the gut microbiome impact brain pathophysiology in T2DM remain unexplored. On this basis, in male Zucker diabetic fatty (ZDF) rats, we studied whether T2DM alters memory abilities using the novel object recognition test, neuroactive steroid levels by liquid chromatography–tandem mass spectrometry, hippocampal parameters using molecular assessments, and gut microbiome composition using 16S next-generation sequencing. Results obtained reveal that T2DM worsens memory abilities and that these are correlated with increased levels of corticosterone in plasma and with a decrease in allopregnanolone in the hippocampus, where neuroinflammation, oxidative stress, and mitochondrial dysfunction were reported. Interestingly, our analysis highlighted a small group of taxa strictly related to both memory impairment and neuroactive steroid levels. Overall, the data underline an interesting role for allopregnanolone and microbiota that may represent candidates for the development of therapeutic strategies.
Full article
(This article belongs to the Special Issue Role of Neuroactive Steroids in Health and Disease)
►▼
Show Figures
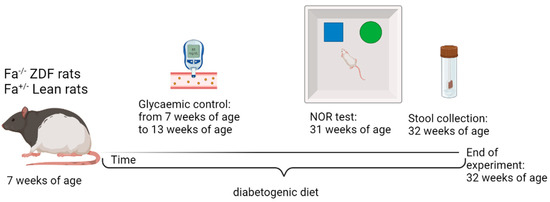
Figure 1
Open AccessArticle
Low-Dose Chidamide Treatment Displays Sex-Specific Differences in the 3xTg-AD Mouse
by
, , , , and
Biomolecules 2023, 13(9), 1324; https://doi.org/10.3390/biom13091324 - 29 Aug 2023
Abstract
Epigenetic compounds have become attractive small molecules for targeting the multifaceted aspects of Alzheimer’s disease (AD). Although AD disproportionately affects women, most of the current literature investigating epigenetic compounds for the treatment of AD do not report sex-specific results. This is remarkable because
[...] Read more.
Epigenetic compounds have become attractive small molecules for targeting the multifaceted aspects of Alzheimer’s disease (AD). Although AD disproportionately affects women, most of the current literature investigating epigenetic compounds for the treatment of AD do not report sex-specific results. This is remarkable because there is rising evidence that epigenetic compounds intrinsically affect males and females differently. This manuscript explores the sexual dimorphism observed after chronic, low-dose administration of a clinically relevant histone deacetylase inhibitor, chidamide (Tucidinostat), in the 3xTg-AD mouse model. We found that chidamide treatment significantly improves glucose tolerance and increases expression of glucose transporters in the brain of males. We also report a decrease in total tau in chidamide-treated mice. Differentially expressed genes in chidamide-treated mice were much greater in males than females. Genes involved in the neuroinflammatory pathway and amyloid processing pathway were mostly upregulated in chidamide-treated males while downregulated in chidamide-treated females. This work highlights the need for drug discovery projects to consider sex as a biological variable to facilitate translation.
Full article
(This article belongs to the Special Issue From Biomarkers to Therapy to Puzzle Out Alzheimer’s Disease)
►▼
Show Figures
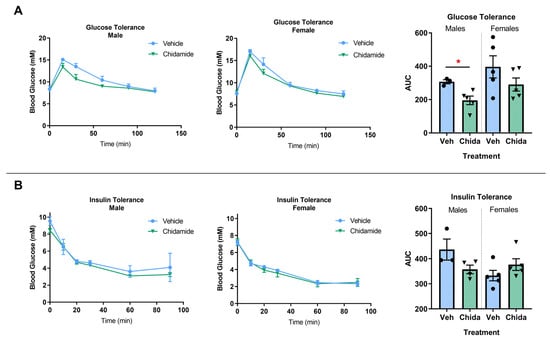
Figure 1
Open AccessArticle
Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients
by
, , , , , , , , and
Biomolecules 2023, 13(9), 1323; https://doi.org/10.3390/biom13091323 - 29 Aug 2023
Abstract
Considering the relationship between disease severity and the extent of metabolic derangement in heart failure, we hypothesized that the serum levels of metabolites may have prognostic value for 1-year mortality in acute heart failure (AHF). The AHF study was a prospective, observational study
[...] Read more.
Considering the relationship between disease severity and the extent of metabolic derangement in heart failure, we hypothesized that the serum levels of metabolites may have prognostic value for 1-year mortality in acute heart failure (AHF). The AHF study was a prospective, observational study enrolling consecutive patients hospitalized due to AHF. Metabolites were measured in serum collected at admission using NMR spectroscopy. Out of 315 AHF patients, 118 (37.5%) died within 1 year after hospitalization for AHF. The serum levels of 8 out of 49 identified metabolites were significantly different between patients who were alive and those who died within 1 year after hospitalization for AHF. Of these, only valine was significantly associated with 1-year mortality (hazard ratio 0.73 per 1 standard deviation increase, 95% confidence interval: 0.59–0.90, p = 0.003) in the multivariable Cox regression analyses. Kaplan–Maier analysis showed significantly higher survival rates in AHF patients with valine levels above the median (>279.2 µmol/L) compared to those with valine levels ≤ 279.2 µmol/L. In a receiver operating characteristics curve analysis, valine was able to discriminate between the two groups with an area under the curve of 0.65 (95% CI 0.59–0.72). We conclude that valine serum levels might be of prognostic value in AHF.
Full article
(This article belongs to the Special Issue Biomarkers Profile and Biomolecular Signaling in Heart Failure and Cardiomyopathies)
►▼
Show Figures
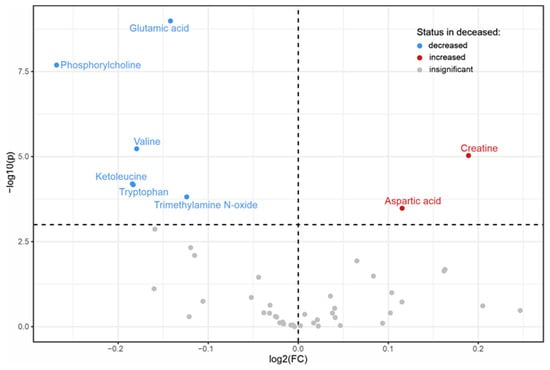
Figure 1
Open AccessArticle
In Silico Analysis of Pyeongwi-San Involved in Inflammatory Bowel Disease Treatment Using Network Pharmacology, Molecular Docking, and Molecular Dynamics
Biomolecules 2023, 13(9), 1322; https://doi.org/10.3390/biom13091322 - 28 Aug 2023
Abstract
Backgound: Pyeongwi-san (PWS) is a widely used formula for treating digestive disorders in Korea and China. Inflammatory bowel disease (IBD) is characterized by progressive inflammation of the gastrointestinal tract. Emerging evidence supports the protective effect of PWS against IBD, but specific mechanisms are
[...] Read more.
Backgound: Pyeongwi-san (PWS) is a widely used formula for treating digestive disorders in Korea and China. Inflammatory bowel disease (IBD) is characterized by progressive inflammation of the gastrointestinal tract. Emerging evidence supports the protective effect of PWS against IBD, but specific mechanisms are still elusive. Methods: Active compounds of PWS were screened from the medicinal materials and chemical compounds in Northeast Asian traditional medicine (TM-MC) in the consideration of drug-likeness and oral bioavailability. Target candidates of active compounds were predicted using the ChEMBL database. IBD-related targets were obtained from the GeneCards and DisGeNET databases. The network of composition-targets-disease was constructed. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were analyzed. Molecular docking was used to simulate the binding affinity of active compounds on target proteins and molecular dynamics was used to validate the molecular docking result. Results: A total of 26 core target proteins of PWS were related to IBD. Enrichment analysis suggested that PWS is highly associated with tumor necrosis factor signaling pathway, apoptosis, and the collapse of tight junctions. Moreover, molecular docking and molecular dynamics simulation proposed β-eudesmol and (3R,6R,7S)-1,10-bisaboladien-3-ol to ameliorate IBD through the binding to TNF and MMP9, respectively. Conclusion: Present in silico analysis revealed potential pathways and insight of PWS to regulate IBD. These results imply that the therapeutic effect of PWS might be achieved via an inhibitory effect.
Full article
(This article belongs to the Special Issue Phytochemical Constituents and Medicinal Plants for the Treatment of Inflammatory Diseases in 2023)
►▼
Show Figures
Figure 1
Open AccessArticle
Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis
Biomolecules 2023, 13(9), 1321; https://doi.org/10.3390/biom13091321 - 28 Aug 2023
Abstract
The concurrent use of several medications is a common practice in the treatment of complex psychiatric conditions. One such commonly used combination is aripiprazole (ARI), an antipsychotic, and trazodone (TRZ), an antidepressant. In addition to their effects on dopamine and serotonin systems, both
[...] Read more.
The concurrent use of several medications is a common practice in the treatment of complex psychiatric conditions. One such commonly used combination is aripiprazole (ARI), an antipsychotic, and trazodone (TRZ), an antidepressant. In addition to their effects on dopamine and serotonin systems, both of these compounds are inhibitors of the 7-dehydrocholesterol reductase (DHCR7) enzyme. To evaluate the systemic and nervous system distribution of ARI and TRZ and their effects on cholesterol biosynthesis, adult mice were treated with both ARI and TRZ for 21 days. The parent drugs, their metabolites, and sterols were analyzed in the brain and various organs of mice using LC-MS/MS. The analyses revealed that ARI, TRZ, and their metabolites were readily detectable in the brain and organs, leading to changes in the sterol profile. The levels of medications, their metabolites, and sterols differed across tissues with notable sex differences. Female mice showed higher turnover of ARI and more cholesterol clearance in the brain, with several post-lanosterol intermediates significantly altered. In addition to interfering with sterol biosynthesis, ARI and TRZ exposure led to decreased ionized calcium-binding adaptor molecule 1 (IBA1) and increased DHCR7 protein expression in the cortex. Changes in sterol profile have been also identified in the spleen, liver, and serum, underscoring the systemic effect of ARI and TRZ on sterol biosynthesis. Long-term use of concurrent ARI and TRZ warrants further studies to fully evaluate the lasting consequences of altered sterol biosynthesis on the whole body.
Full article
(This article belongs to the Special Issue Brain Sterols: Biosynthesis and Physiology in Health and Disease)
►▼
Show Figures

Figure 1
Open AccessArticle
Diabetes-Induced Amplification of Nociceptive DRG Neuron Output by Upregulation of Somatic T-Type Ca2+ Channels
by
, , , , , and
Biomolecules 2023, 13(9), 1320; https://doi.org/10.3390/biom13091320 - 28 Aug 2023
Abstract
The development of pain symptoms in peripheral diabetic neuropathy (PDN) is associated with the upregulation of T-type Ca2+ channels (T-channels) in the soma of nociceptive DRG neurons. Moreover, a block of these channels in DRG neurons effectively reversed mechanical and thermal hyperalgesia
[...] Read more.
The development of pain symptoms in peripheral diabetic neuropathy (PDN) is associated with the upregulation of T-type Ca2+ channels (T-channels) in the soma of nociceptive DRG neurons. Moreover, a block of these channels in DRG neurons effectively reversed mechanical and thermal hyperalgesia in animal diabetic models, indicating that T-channel functioning in these neurons is causally linked to PDN. However, no particular mechanisms relating the upregulation of T-channels in the soma of nociceptive DRG neurons to the pathological pain processing in PDN have been suggested. Here we have electrophysiologically identified voltage-gated currents expressed in nociceptive DRG neurons and developed a computation model of the neurons, including peripheral and central axons. Simulations showed substantially stronger sensitivity of neuronal excitability to diabetes-induced T-channel upregulation at the normal body temperature compared to the ambient one. We also found that upregulation of somatic T-channels, observed in these neurons under diabetic conditions, amplifies a single action potential invading the soma from the periphery into a burst of multiple action potentials further propagated to the end of the central axon. We have concluded that the somatic T-channel-dependent amplification of the peripheral nociceptive input to the spinal cord demonstrated in this work may underlie abnormal nociception at different stages of diabetes development.
Full article
(This article belongs to the Special Issue Featured Papers in Ion Channels Diseases)
►▼
Show Figures
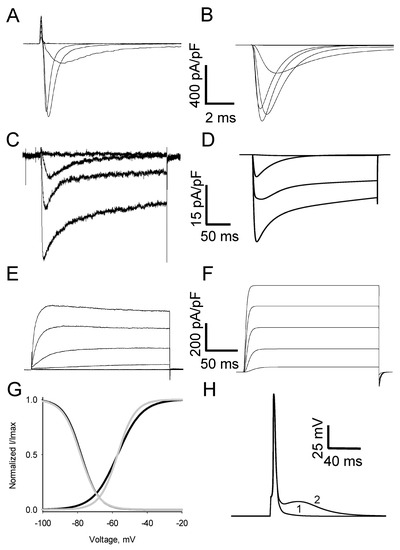
Figure 1
Open AccessReview
Advances in Dystrophinopathy Diagnosis and Therapy
Biomolecules 2023, 13(9), 1319; https://doi.org/10.3390/biom13091319 - 28 Aug 2023
Abstract
Dystrophinopathies are x-linked muscular disorders which emerge from mutations in the Dystrophin gene, including Duchenne and Becker muscular dystrophy, and dilated cardiomyopathy. However, Duchenne muscular dystrophy interconnects with bone loss and osteoporosis, which are exacerbated by glucocorticoids therapy. Procedures for diagnosing dystrophinopathies include
[...] Read more.
Dystrophinopathies are x-linked muscular disorders which emerge from mutations in the Dystrophin gene, including Duchenne and Becker muscular dystrophy, and dilated cardiomyopathy. However, Duchenne muscular dystrophy interconnects with bone loss and osteoporosis, which are exacerbated by glucocorticoids therapy. Procedures for diagnosing dystrophinopathies include creatine kinase assay, haplotype analysis, Southern blot analysis, immunological analysis, multiplex PCR, multiplex ligation-dependent probe amplification, Sanger DNA sequencing, and next generation DNA sequencing. Pharmacological therapy for dystrophinopathies comprises glucocorticoids (prednisone, prednisolone, and deflazacort), vamorolone, and ataluren. However, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and β-blockers are the first-line to prevent dilated cardiomyopathy in dystrophinopathy patients. Duchenne muscular dystrophy gene therapy strategies involve gene transfer, exon skipping, exon reframing, and CRISPR gene editing. Eteplirsen, an antisense-oligonucleotide drug for skipping exon 51 from the Dystrophin gene, is available on the market, which may help up to 14% of Duchenne muscular dystrophy patients. There are various FDA-approved exon skipping drugs including ExonDys-51 for exon 51, VyonDys-53 and Viltolarsen for exon 53 and AmonDys-45 for exon 45 skipping. Other antisense oligonucleotide drugs in the pipeline include casimersen for exon 45, suvodirsen for exon 51, and golodirsen for exon 53 skipping. Advances in the diagnosis and therapy of dystrophinopathies offer new perspectives for their early discovery and care.
Full article
(This article belongs to the Special Issue Molecular Basis and Translational Research in Metabolic Myopathies: A Themed Issue in Honor of Professor Corrado Angelini)
►▼
Show Figures
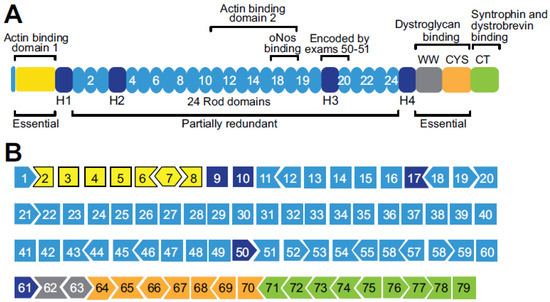
Figure 1

Journal Menu
► ▼ Journal Menu-
- Biomolecules Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biomolecules, Cells, CIMB, IJMS, JMP, Molecules, Proteomes
Metalloproteins and Metalloenzymes
Topic Editors: Eugene A. Permyakov, Ludmilla Morozova-RocheDeadline: 30 September 2023
Topic in
Biology, Biomolecules, Cells, JCM, Organoids
Organ Fibrosis: From Molecular Mechanisms to Clinical Therapies
Topic Editors: Maurizio Onisto, Valentina MasolaDeadline: 31 October 2023
Topic in
Antioxidants, Biomolecules, Molecules, Pharmaceutics, Separations
Application of Analytical Chemistry in Exercise Physiology and Pharmacology
Topic Editors: Andrzej Pokrywka, Dorota KwiatkowskaDeadline: 15 November 2023
Topic in
Biomolecules, Cancers, Cells, IJMS, JCM, JMP, Organoids
Novel Discoveries in Oncology
Topic Editors: Alessia Filippone, Giovanna Casili, Marika Lanza, Michela CampoloDeadline: 20 November 2023

Conferences
Special Issues
Special Issue in
Biomolecules
Molecular Mechanisms and Innovative Therapies for Cardioprotection
Guest Editors: Marco Piccoli, Montserrat Climent SalarichDeadline: 3 September 2023
Special Issue in
Biomolecules
Role of Connexins in Hereditary Diseases
Guest Editor: Mona M. FreidinDeadline: 15 September 2023
Special Issue in
Biomolecules
Proton and Proton-Coupled Transport
Guest Editor: Peter PohlDeadline: 30 September 2023
Special Issue in
Biomolecules
Redox Imbalance and Mitochondrial Abnormalities in Kidney Disease II
Guest Editor: Liang-Jun YanDeadline: 15 October 2023
Topical Collections
Topical Collection in
Biomolecules
Feature Papers in Molecular Genetics
Collection Editor: Jürg Bähler
Topical Collection in
Biomolecules
Feature Papers in Bioinformatics and Systems Biology Section
Collection Editor: Lukasz Kurgan
Topical Collection in
Biomolecules
Molecular Mechanisms of Obesity, Diabetes, Inflammation and Aging
Collection Editors: Yuxiang Sun, Susanne Talcott