-
 Dendrimers and Derivatives as Multifunctional Nanotherapeutics for Alzheimer’s Disease
Dendrimers and Derivatives as Multifunctional Nanotherapeutics for Alzheimer’s Disease -
 Layer-by-Layer Nanoassemblies for Vaccination Purposes
Layer-by-Layer Nanoassemblies for Vaccination Purposes -
 Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review
Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review -
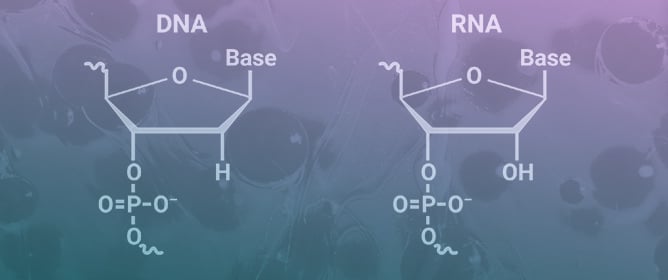 Enhancing the Effectiveness of Oligonucleotide Therapeutics Using Cell-Penetrating Peptide Conjugation, Chemical Modification, and Carrier-Based Delivery Strategies
Enhancing the Effectiveness of Oligonucleotide Therapeutics Using Cell-Penetrating Peptide Conjugation, Chemical Modification, and Carrier-Based Delivery Strategies -
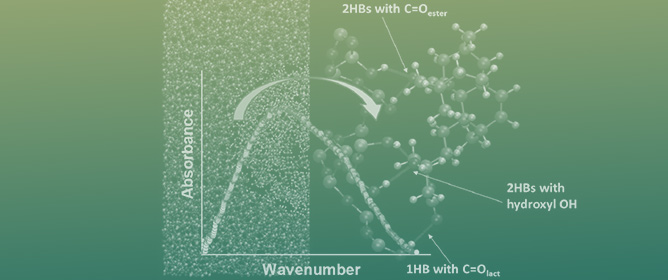 Evidence of Strong Guest–Host Interactions in Simvastatin Loaded in Mesoporous Silica MCM-41
Evidence of Strong Guest–Host Interactions in Simvastatin Loaded in Mesoporous Silica MCM-41
Journal Description
Pharmaceutics
Pharmaceutics
is a peer-reviewed, open access journal on the science and technology of pharmaceutics and biopharmaceutics, and is published monthly online by MDPI. The Spanish Society of Pharmaceutics and Pharmaceutical Technology (SEFIG), Pharmaceutical Solid State Research Cluster (PSSRC), Academy of Pharmaceutical Sciences (APS) and Korean Society of Pharmaceutical Sciences and Technology (KSPST) are affiliated with Pharmaceutics and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology & Pharmacy) / CiteScore - Q1 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 17 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the first half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Companion journal: Future Pharmacology
Impact Factor:
5.4 (2022);
5-Year Impact Factor:
6.0 (2022)
Latest Articles
Comprehensive Assessment of Pharmacokinetics, Pharmacodynamics, and Tolerability of Ligelizumab in Healthy Volunteers and Patients with Chronic Spontaneous Urticaria to Optimize Its Subcutaneous Delivery System
Pharmaceutics 2023, 15(9), 2266; https://doi.org/10.3390/pharmaceutics15092266 (registering DOI) - 01 Sep 2023
Abstract
Ligelizumab is a highly potent, humanized IgG1, anti-IgE monoclonal antibody. To explore its optimal subcutaneous delivery, the pharmacokinetics (PK), pharmacodynamics (PD), and tolerability of ligelizumab from two Phase 1 studies in healthy volunteers (HVs) and four Phase 2 and 3 studies in patients
[...] Read more.
Ligelizumab is a highly potent, humanized IgG1, anti-IgE monoclonal antibody. To explore its optimal subcutaneous delivery, the pharmacokinetics (PK), pharmacodynamics (PD), and tolerability of ligelizumab from two Phase 1 studies in healthy volunteers (HVs) and four Phase 2 and 3 studies in patients with chronic spontaneous urticaria (CSU) were assessed. Using different injection volumes or durations of a liquid-in-vial (LIVI) formulation or different formulations (LIVI vs. prefilled syringe (PFS)), single-dose ligelizumab showed comparable PK exposure in HVs. Steady-state exposure of ligelizumab was also comparable between LIVI and PFS following multiple dosing in CSU patients. The total IgE level (a PD marker) and tolerability were similar between the two formulations in both HVs and patients. Furthermore, the PK, total IgE, and tolerability were comparable for PFS administered either by patients or healthcare providers (HCPs). Collective evidence demonstrated that the injection duration or volume, formulation, or administrator had no apparent impact on the PK, PD, and tolerability of ligelizumab, supporting no clinically relevant difference between LIVI and PFS, and that PFS can be administered by patients or HCPs. This report provides a comprehensive assessment based on data of multiple clinical endpoints from both HVs and patients to inform formulation development and commercial use of a monoclonal antibody.
Full article
(This article belongs to the Special Issue Role of Pharmacokinetics in Drug Development and Evaluation)
►
Show Figures
Open AccessCommunication
Concept for a Unidirectional Release Mucoadhesive Buccal Tablet for Oral Delivery of Antidiabetic Peptide Drugs Such as Insulin, Glucagon-like Peptide 1 (GLP-1), and their Analogs
Pharmaceutics 2023, 15(9), 2265; https://doi.org/10.3390/pharmaceutics15092265 (registering DOI) - 01 Sep 2023
Abstract
Injectable peptides such as insulin, glucagon-like peptide 1 (GLP-1), and their agonists are being increasingly used for the treatment of diabetes. Currently, the most common route of administration is injection, which is linked to patient discomfort as well as being subjected to refrigerated
[...] Read more.
Injectable peptides such as insulin, glucagon-like peptide 1 (GLP-1), and their agonists are being increasingly used for the treatment of diabetes. Currently, the most common route of administration is injection, which is linked to patient discomfort as well as being subjected to refrigerated storage and the requirement for efficient supply chain logistics. Buccal and sublingual routes are recognized as valid alternatives due to their high accessibility and easy administration. However, there can be several challenges, such as peptide selection, drug encapsulation, and delivery system design, which are linked to the enhancement of drug efficacy and efficiency. By using hydrophobic polymers that do not dissolve in saliva, and by using neutral or positively charged nanoparticles that show better adhesion to the negative charges generated by the sialic acid in the mucus, researchers have attempted to improve drug efficiency and efficacy in buccal delivery. Furthermore, unidirectional films and tablets seem to show the highest bioavailability as compared to sprays and other buccal delivery vehicles. This advantageous attribute can be attributed to their capability to mitigate the impact of saliva and inadvertent gastrointestinal enzymatic digestion, thereby minimizing drug loss. This is especially pertinent as these formulations ensure a more directed drug delivery trajectory, leading to heightened therapeutic outcomes. This communication describes the current state of the art with respect to the creation of nanoparticles containing peptides such as insulin, glucagon-like peptide 1 (GLP-1), and their agonists, and theorizes the production of mucoadhesive unidirectional release buccal tablets or films. Such an approach is more patient-friendly and can improve the lives of millions of diabetics around the world; in addition, these shelf-stable formulations ena a more environmentally friendly and sustainable supply chain network.
Full article
(This article belongs to the Special Issue Micronutrient Delivery Systems: Potential Health-Promoting Applications)
►▼
Show Figures
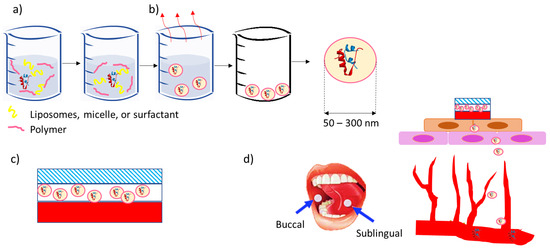
Figure 1
Open AccessArticle
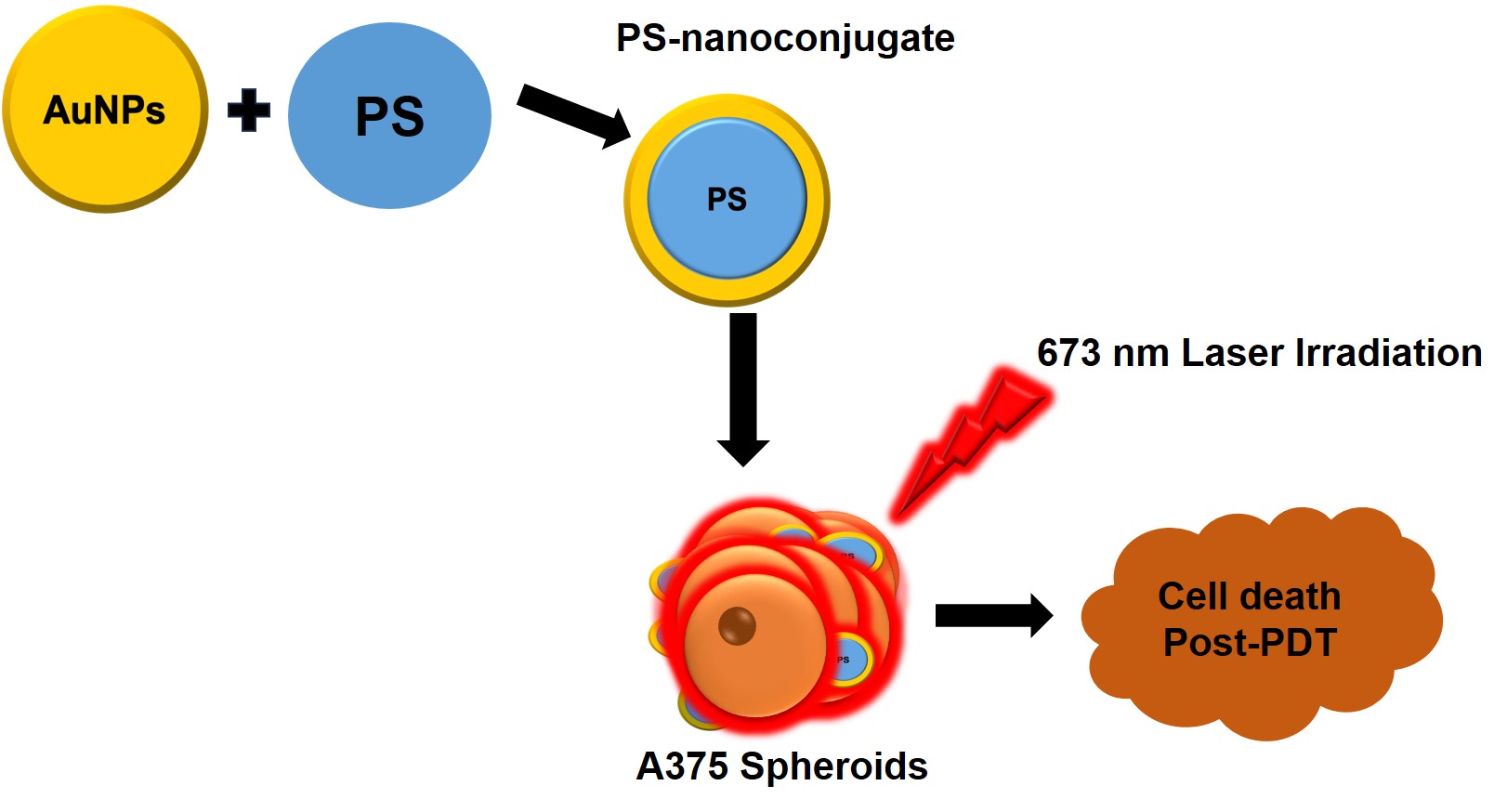
The Efficacy of Zinc Phthalocyanine Nanoconjugate on Melanoma Cells Grown as Three-Dimensional Multicellular Tumour Spheroids
Pharmaceutics 2023, 15(9), 2264; https://doi.org/10.3390/pharmaceutics15092264 (registering DOI) - 31 Aug 2023
Abstract
Melanoma remains a major public health concern that is highly resistant to standard therapeutic approaches. Photodynamic therapy (PDT) is an underutilised cancer therapy with an increased potency and negligible side effects, and it is non-invasive compared to traditional treatment modalities. Three-dimensional multicellular tumour
[...] Read more.
Melanoma remains a major public health concern that is highly resistant to standard therapeutic approaches. Photodynamic therapy (PDT) is an underutilised cancer therapy with an increased potency and negligible side effects, and it is non-invasive compared to traditional treatment modalities. Three-dimensional multicellular tumour spheroids (MCTS) closely resemble in vivo avascular tumour features, allowing for the more efficient and precise screening of novel anticancer agents with various treatment combinations. In this study, we utilised A375 human melanoma spheroids to screen the phototoxic effect of zinc phthalocyanine tetrasulfonate (ZnPcS4) conjugated to gold nanoparticles (AuNP). The nanoconjugate was synthesised and characterised using ultraviolet-visible spectroscopy, a high-resolution transmission electron microscope (TEM), dynamic light scattering (DLS), and zeta potential (ZP). The phototoxicity of the nanoconjugate was tested on the A375 MCTS using PDT at a fluency of 10 J/cm2. After 24 h, the cellular responses were evaluated via microscopy, an MTT viability assay, an ATP luminescence assay, and cell death induction using annexin propidium iodide. The MTT viability assay demonstrated that the photoactivated ZnPcS4, at a concentration of 12.73 µM, caused an approximately 50% reduction in the cell viability of the spheroids. When conjugated to AuNPs, the latter significantly increased the cellular uptake and cytotoxicity in the melanoma spheroids via the induction of apoptosis. This novel Zinc Phthalocyanine Nanoconjugate shows promise as a more effective PDT treatment modality.
Full article
(This article belongs to the Special Issue Advances in Cancer Nanotechnology for Photodynamic and Photothermal Therapy)
►▼
Show Figures
Graphical abstract
Open AccessArticle
A Species-Specific Anti-Human P2X7 Monoclonal Antibody Reduces Graft-versus-Host Disease in Humanised Mice
Pharmaceutics 2023, 15(9), 2263; https://doi.org/10.3390/pharmaceutics15092263 (registering DOI) - 31 Aug 2023
Abstract
Graft-versus-host disease (GVHD) is a T cell-mediated inflammatory disorder that arises from allogeneic haematopoietic stem cell transplantation and is often fatal. The P2X7 receptor is an extracellular adenosine 5′-triphosphate-gated cation channel expressed on immune cells. Blockade of this receptor with small molecule inhibitors
[...] Read more.
Graft-versus-host disease (GVHD) is a T cell-mediated inflammatory disorder that arises from allogeneic haematopoietic stem cell transplantation and is often fatal. The P2X7 receptor is an extracellular adenosine 5′-triphosphate-gated cation channel expressed on immune cells. Blockade of this receptor with small molecule inhibitors impairs GVHD in a humanised mouse model. A species-specific blocking monoclonal antibody (mAb) (clone L4) for human P2X7 is available, affording the opportunity to determine whether donor (human) P2X7 contributes to the development of GVHD in humanised mice. Using flow cytometric assays of human RPMI 8266 and murine J774 cells, this study confirmed that this mAb bound and impaired human P2X7. Furthermore, this mAb prevented the loss of human regulatory T cells (hTregs) and natural killer (hNK) T cells in vitro. NOD-scid IL2Rγnull mice were injected with 10 × 106 human peripheral blood mononuclear cells (Day 0) and an anti-hP2X7 or control mAb (100 μg i.p. per mouse, Days 0, 2, 4, 6, and 8). The anti-hP2X7 mAb increased hTregs and hNK cells at Day 21. Moreover, anti-hP2X7 mAb-treatment reduced clinical and histological GVHD in the liver and lung compared to the control treatment at disease endpoint. hTregs, hNK, and hNK T cell proportions were increased, and human T helper 17 cell proportions were decreased at endpoint. These studies indicate that blockade of human (donor) P2X7 reduces GVHD development in humanised mice, providing the first direct evidence of a role for donor P2X7 in GVHD.
Full article
(This article belongs to the Section Biologics and Biosimilars)
►▼
Show Figures
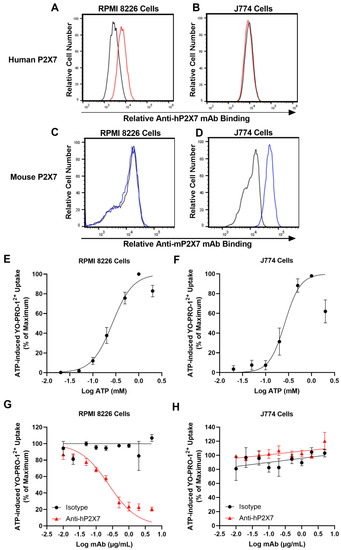
Figure 1
Open AccessEditorial
Radiopharmaceuticals for Cancer Imaging and Therapy
by
and
Pharmaceutics 2023, 15(9), 2262; https://doi.org/10.3390/pharmaceutics15092262 (registering DOI) - 31 Aug 2023
Abstract
Nuclear medicine has emerged as a pivotal player in cancer patient care, revolutionizing the way cancer is detected, diagnosed, monitored, and treated [...]
Full article
(This article belongs to the Special Issue Radiopharmaceuticals for Cancer Imaging and Therapy)
Open AccessArticle
Biomimetic Electrospun Self-Assembling Peptide Scaffolds for Neural Stem Cell Transplantation in Neural Tissue Engineering
by
, , , , and
Pharmaceutics 2023, 15(9), 2261; https://doi.org/10.3390/pharmaceutics15092261 (registering DOI) - 31 Aug 2023
Abstract
Spinal cord regeneration using stem cell transplantation is a promising strategy for regenerative therapy. Stem cells transplanted onto scaffolds that can mimic natural extracellular matrix (ECM) have the potential to significantly improve outcomes. In this study, we strived to develop a cell carrier
[...] Read more.
Spinal cord regeneration using stem cell transplantation is a promising strategy for regenerative therapy. Stem cells transplanted onto scaffolds that can mimic natural extracellular matrix (ECM) have the potential to significantly improve outcomes. In this study, we strived to develop a cell carrier by culturing neural stem cells (NSCs) onto electrospun 2D and 3D constructs made up of specific crosslinked functionalized self-assembling peptides (SAPs) featuring enhanced biomimetic and biomechanical properties. Morphology, architecture, and secondary structures of electrospun scaffolds in the solid-state and electrospinning solution were studied step by step. Morphological studies showed the benefit of mixed peptides and surfactants as additives to form thinner, uniform, and defect-free fibers. It has been observed that β-sheet conformation as evidence of self-assembling has been predominant throughout the process except for the electrospinning solution. In vitro NSCs seeded on electrospun SAP scaffolds in 2D and 3D conditions displayed desirable proliferation, viability, and differentiation in comparison to the gold standard. In vivo biocompatibility assay confirmed the permissibility of implanted fibrous channels by foreign body reaction. The results of this study demonstrated that fibrous 2D/3D electrospun SAP scaffolds, when shaped as micro-channels, can be suitable to support NSC transplantation for regeneration following spinal cord injury.
Full article
(This article belongs to the Special Issue Nanofibrous Scaffolds Application in Biomedicine)
►▼
Show Figures
Figure 1
Open AccessArticle
A Novel Formulation of Asparaginase Encapsulated into Virus-like Particles of Brome Mosaic Virus: In Vitro and In Vivo Evidence
Pharmaceutics 2023, 15(9), 2260; https://doi.org/10.3390/pharmaceutics15092260 (registering DOI) - 31 Aug 2023
Abstract
The interest in plant-derived virus-like particles (pVLPs) for the design of a new generation of nanocarriers is based on their lack of infection for humans, their immunostimulatory properties to fight cancer cells, and their capability to contain and release cargo molecules. Asparaginase (ASNase)
[...] Read more.
The interest in plant-derived virus-like particles (pVLPs) for the design of a new generation of nanocarriers is based on their lack of infection for humans, their immunostimulatory properties to fight cancer cells, and their capability to contain and release cargo molecules. Asparaginase (ASNase) is an FDA-approved drug to treat acute lymphoblastic leukemia (LLA); however, it exhibits high immunogenicity which often leads to discontinuation of treatment. In previous work, we encapsulated ASNase into bacteriophage P22-based VLPs through genetic-directed design to form the ASNase-P22 nanobioreactors. In this work, a commercial ASNase was encapsulated into brome mosaic virus-like particles (BMV-VLPs) to form stable ASNase-BMV nanobioreactors. According to our results, we observed that ASNase-BMV nanobioreactors had similar cytotoxicity against MOLT-4 and Reh cells as the commercial drug. In vivo assays showed a higher specific anti-ASNase IgG response in BALB/c mice immunized with ASNase encapsulated into BMV-VLPs compared with those immunized with free ASNase. Nevertheless, we also detected a high and specific IgG response against BMV capsids on both ASNase-filled capsids (ASNase-BMV) and empty BMV capsids. Despite the fact that our in vivo studies showed that the BMV-VLPs stimulate the immune response either empty or with cargo proteins, the specific cytotoxicity against leukemic cells allows us to propose ASNase-BMV as a potential novel formulation for LLA treatment where in vitro and in vivo evidence of functionality is provided.
Full article
(This article belongs to the Special Issue Nanocarriers for Cancer Therapy and Diagnosis, 2nd Edition)
►▼
Show Figures
Figure 1
Open AccessArticle
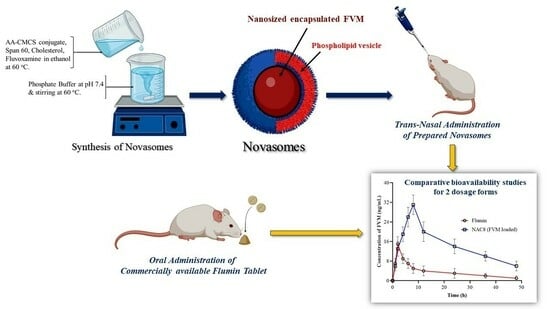
Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate
by
, , , , , , , , , , , , , , and
Pharmaceutics 2023, 15(9), 2259; https://doi.org/10.3390/pharmaceutics15092259 (registering DOI) - 31 Aug 2023
Abstract
Depression is the major mental illness which causes along with loss of interest in daily life, a feeling of hopelessness, appetite or weight changes, anger and irritability. Due to the hepatic first-pass metabolism, the absolute bioavailability of fluvoxamine (FVM) after oral administration is
[...] Read more.
Depression is the major mental illness which causes along with loss of interest in daily life, a feeling of hopelessness, appetite or weight changes, anger and irritability. Due to the hepatic first-pass metabolism, the absolute bioavailability of fluvoxamine (FVM) after oral administration is about 50%. By avoiding the pre-systemic metabolism, nasal delivery would boost bioavailability of FVM. Additionally, the absorption is anticipated to occur more quickly than it would via the oral route because of the existence of microvilli and high vasculature. A nonionic surfactant, cholesterol and an arachidonic acid-carboxymethyl chitosan (AA-CMCS) conjugate were used to develop FVM-loaded novasomes. To investigate the effects of surfactant concentration, AA-CMCS conjugate concentration and stirring speed on the novasomes’ characteristics, a Box–Behnken design was used. The dependent variables chosen were zeta potential, polydispersity index and particle size. The AA-CMCS conjugate was confirmed by 1H-NMR and FTIR. Using Design Expert software (version 7; Stat-Ease Inc., Minneapolis, MN, USA), novasomes were further optimized. The chosen optimal formulation (NAC8) was made up of AA-CMCS conjugate, Span 60 and cholesterol. Particle size, zeta potential and PDI values for NAC8 formulation were 101 nm, −35 mV and 0.263, respectively. The NAC8 formulation’s DSC and TGA analysis demonstrated that the medication had been uniformly and amorphously distributed throughout the novasomes. The NAC8 formulation showed 99% and 90% FVM release and permeation, respectively, and the novasome adherence time was 24 h. An improved antidepressant effect along with five-fold increase in bioavailability of FVM was observed after trans-nasal administration of NAC8 formulation compared to the reference commercially available Flumin® tablets. FVM-loaded novasomes administered via the nasal route may therefore constitute an advancement in the management of depression.
Full article
(This article belongs to the Special Issue Functional Nanomaterials for Drug Delivery and Pharmaceutical Applications)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Mesoporous Composite Bioactive Compound Delivery System for Wound-Healing Processes
by
, , , , , , , , , , and
Pharmaceutics 2023, 15(9), 2258; https://doi.org/10.3390/pharmaceutics15092258 (registering DOI) - 31 Aug 2023
Abstract
Currently, the treatment of wounds is still a challenge for healthcare professionals due to high complication incidences and social impacts, and the development of biocompatible and efficient medicines remains a goal. In this regard, mesoporous materials loaded with bioactive compounds from natural extracts
[...] Read more.
Currently, the treatment of wounds is still a challenge for healthcare professionals due to high complication incidences and social impacts, and the development of biocompatible and efficient medicines remains a goal. In this regard, mesoporous materials loaded with bioactive compounds from natural extracts have a high potential for wound treatment due to their nontoxicity, high loading capacity and slow drug release. MCM-41-type mesoporous material was synthesized by using sodium trisilicate as a silica source at room temperature and normal pressure. The synthesized mesoporous silica was characterized by using Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), N2 absorption–desorption (BET), Dynamic Light Scattering (DLS) and Fourier transform infrared spectroscopy (FT-IR), revealing a high surface area (BET, 1244 m2/g); pore diameter of approx. 2 nm; and a homogenous, ordered and hexagonal geometry (TEM images). Qualitative monitoring of the desorption degree of the Salvia officinalis (SO) extract, rich in ursolic acid and oleanolic acid, and Calendula officinalis (CO) extract, rich in polyphenols and flavones, was performed via the continuous recording of the UV-VIS spectra at predetermined intervals. The active ingredients in the new composite MCM-41/sage and marigold (MCM-41/SO&CO) were quantified by using HPLC-DAD and LC-MS-MS techniques. The evaluation of the biological composites’ activity on the wound site was performed on two cell lines, HS27 and HaCaT, naturally involved in tissue-regeneration processes. The experimental results revealed the ability to stimulate collagen biosynthesis, the enzymatic activity of the main metalloproteinases (MMP-2 and MMP-9) involved in tissue remodeling processes and the migration rate in the wound site, thus providing insights into the re-epithelializing properties of mesoporous composites.
Full article
(This article belongs to the Section Drug Delivery and Controlled Release)
►▼
Show Figures

Figure 1
Open AccessReview
Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials
by
and
Pharmaceutics 2023, 15(9), 2257; https://doi.org/10.3390/pharmaceutics15092257 - 31 Aug 2023
Abstract
Photodynamic therapy (PDT) is an emerging and less invasive treatment modality for various types of cancer. This review provides an overview of recent trends in PDT research, ranging from basic research to ongoing clinical trials, focusing on different cancer types. Lung cancer, head
[...] Read more.
Photodynamic therapy (PDT) is an emerging and less invasive treatment modality for various types of cancer. This review provides an overview of recent trends in PDT research, ranging from basic research to ongoing clinical trials, focusing on different cancer types. Lung cancer, head and neck cancer, non-melanoma skin cancer, prostate cancer, and breast cancer are discussed in this context. In lung cancer, porfimer sodium, chlorin e6, and verteporfin have shown promising results in preclinical studies and clinical trials. For head and neck cancer, PDT has demonstrated effectiveness as an adjuvant treatment after surgery. PDT with temoporfin, redaporfin, photochlor, and IR700 shows potential in early stage larynx cancer and recurrent head and neck carcinoma. Non-melanoma skin cancer has been effectively treated with PDT using methyl aminolevulinate and 5-aminolevulinic acid. In prostate cancer and breast cancer, PDT research is focused on developing targeted photosensitizers to improve tumor-specific uptake and treatment response. In conclusion, PDT continues to evolve as a promising cancer treatment strategy, with ongoing research spanning from fundamental investigations to clinical trials, exploring various photosensitizers and treatment combinations. This review sheds light on the recent advancements in PDT for cancer therapy and highlights its potential for personalized and targeted treatments.
Full article
(This article belongs to the Special Issue Advances in Phototherapy and Sonodynamic Therapy)
►▼
Show Figures
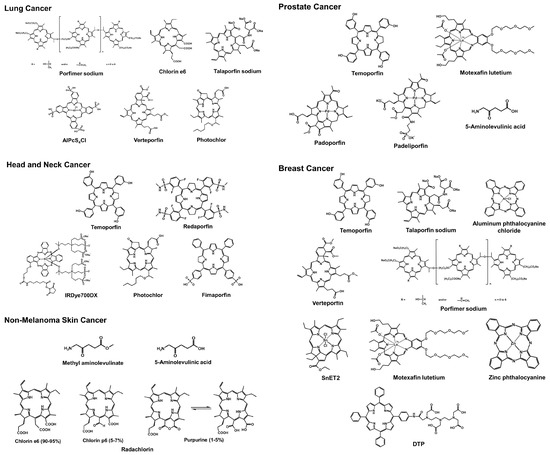
Figure 1
Open AccessArticle
Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning
Pharmaceutics 2023, 15(9), 2256; https://doi.org/10.3390/pharmaceutics15092256 - 31 Aug 2023
Abstract
Aceclofenac-loaded polyvinylpyrrolidone fiber-based amorphous solid dispersion was produced successfully by centrifugal spinning. The solution concentration and rotational speed were optimized to produce the fiber-based drug carrier system, with a determined production rate of 12.7 g/h dry solid fibers. The obtained fibers were bead-free
[...] Read more.
Aceclofenac-loaded polyvinylpyrrolidone fiber-based amorphous solid dispersion was produced successfully by centrifugal spinning. The solution concentration and rotational speed were optimized to produce the fiber-based drug carrier system, with a determined production rate of 12.7 g/h dry solid fibers. The obtained fibers were bead-free and smooth-surfaced with an average diameter of 7.5 ± 2.5 μm. Gas chromatographic determinations revealed that ethanol, as a residual solvent, was well below the regulatory limit of 0.5%. Differential scanning calorimetric investigation and infrared spectroscopic measurements were used to track the physicochemical changes that intervene during fiber formation in the solid state. The results revealed that the rapid evaporation of the solvent was accompanied by a probable crystalline to amorphous transition of the active substance during centrifugal spinning. In vitro dissolution studies revealed an instantaneous disintegration of the fibrous structure and a rapid release of the active substance, with the microfibrous webs greatly outperforming the crystalline active substance, especially in the early time-points. This implies that centrifugal spinning offers a viable scale-up production process to prepare drug-loaded fiber-based solid dispersions.
Full article
(This article belongs to the Special Issue Solid Dispersions for Bioavailability Enhancement)
►▼
Show Figures
Figure 1
Open AccessArticle
A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors
by
, , , , , , , , , , , , and
Pharmaceutics 2023, 15(9), 2255; https://doi.org/10.3390/pharmaceutics15092255 - 31 Aug 2023
Abstract
Systemically administered chemotherapy reduces the efficiency of the anticancer agent at the target tumor tissue and results in distributed drug to non-target organs, inducing negative side effects commonly associated with chemotherapy and necessitating repeated administration. Injectable hydrogels present themselves as a potential platform
[...] Read more.
Systemically administered chemotherapy reduces the efficiency of the anticancer agent at the target tumor tissue and results in distributed drug to non-target organs, inducing negative side effects commonly associated with chemotherapy and necessitating repeated administration. Injectable hydrogels present themselves as a potential platform for non-invasive local delivery vehicles that can serve as a slow-releasing drug depot that fills tumor vasculature, tissue, or resection cavities. Herein, we have systematically formulated and tested an injectable shear-thinning hydrogel (STH) with a highly manipulable release profile for delivering doxorubicin, a common chemotherapeutic. By detailed characterization of the STH physical properties and degradation and release dynamics, we selected top candidates for testing in cancer models of increasing biomimicry. Two-dimensional cell culture, tumor-on-a-chip, and small animal models were used to demonstrate the high anticancer potential and reduced systemic toxicity of the STH that exhibits long-term (up to 80 days) doxorubicin release profiles for treatment of breast cancer and glioblastoma. The drug-loaded STH injected into tumor tissue was shown to increase overall survival in breast tumor- and glioblastoma-bearing animal models by 50% for 22 days and 25% for 52 days, respectively, showing high potential for localized, less frequent treatment of oncologic disease with reduced dosage requirements.
Full article
(This article belongs to the Special Issue Advanced Bio-Hybrid Materials for Drug Delivery Systems - New Trends and Perspectives)
►▼
Show Figures
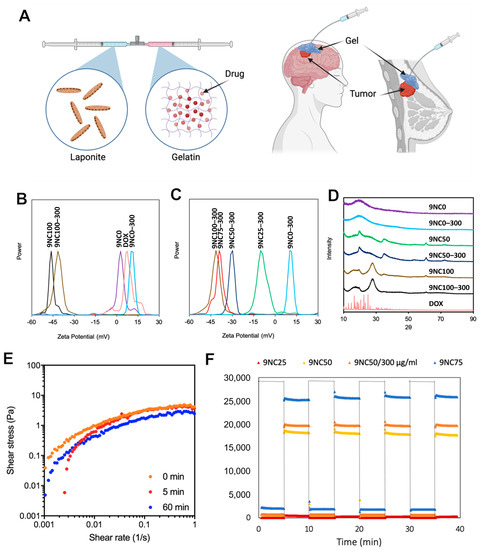
Figure 1
Open AccessReview
Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases
by
, , , , , , and
Pharmaceutics 2023, 15(9), 2254; https://doi.org/10.3390/pharmaceutics15092254 - 31 Aug 2023
Abstract
Inhibitors of phosphodiesterase-4 (PDE4) are small-molecule drugs that, by increasing the intracellular levels of cAMP in immune cells, elicit a broad spectrum of anti-inflammatory effects. As such, PDE4 inhibitors are actively studied as therapeutic options in a variety of human diseases characterized by
[...] Read more.
Inhibitors of phosphodiesterase-4 (PDE4) are small-molecule drugs that, by increasing the intracellular levels of cAMP in immune cells, elicit a broad spectrum of anti-inflammatory effects. As such, PDE4 inhibitors are actively studied as therapeutic options in a variety of human diseases characterized by an underlying inflammatory pathogenesis. Dendritic cells (DCs) are checkpoints of the inflammatory and immune responses, being responsible for both activation and dampening depending on their activation status. This review shows evidence that PDE4 inhibitors modulate inflammatory DC activation by decreasing the secretion of inflammatory and Th1/Th17-polarizing cytokines, although preserving the expression of costimulatory molecules and the CD4+ T cell-activating potential. In addition, DCs activated in the presence of PDE4 inhibitors induce a preferential Th2 skewing of effector T cells, retain the secretion of Th2-attracting chemokines and increase the production of T cell regulatory mediators, such as IDO1, TSP-1, VEGF-A and Amphiregulin. Finally, PDE4 inhibitors selectively induce the expression of the surface molecule CD141/Thrombomodulin/BDCA-3. The result of such fine-tuning is immunomodulatory DCs that are distinct from those induced by classical anti-inflammatory drugs, such as corticosteroids. The possible implications for the treatment of respiratory disorders (such as COPD, asthma and COVID-19) by PDE4 inhibitors will be discussed.
Full article
(This article belongs to the Special Issue Immunotherapeutic Strategies in Cancer and Chronic Infection)
►▼
Show Figures
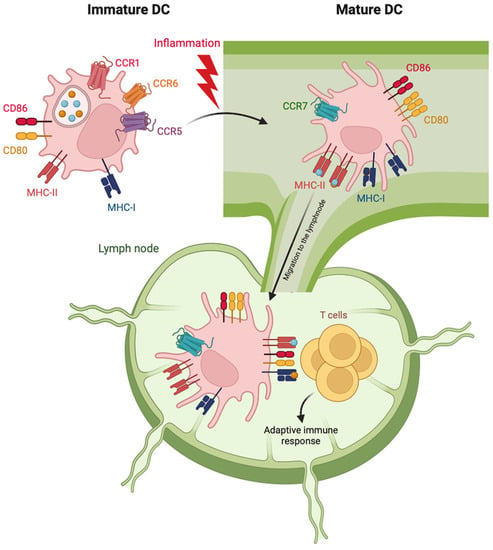
Figure 1
Open AccessReview
Antimicrobial Activity of Selenium Nanoparticles (SeNPs) against Potentially Pathogenic Oral Microorganisms: A Scoping Review
by
, , , , , and
Pharmaceutics 2023, 15(9), 2253; https://doi.org/10.3390/pharmaceutics15092253 - 31 Aug 2023
Abstract
Biofilms are responsible for the most prevalent oral infections such as caries, periodontal disease, and pulp and periapical lesions, which affect the quality of life of people. Antibiotics have been widely used to treat these conditions as therapeutic and prophylactic compounds. However, due
[...] Read more.
Biofilms are responsible for the most prevalent oral infections such as caries, periodontal disease, and pulp and periapical lesions, which affect the quality of life of people. Antibiotics have been widely used to treat these conditions as therapeutic and prophylactic compounds. However, due to the emergence of microbial resistance to antibiotics, there is an urgent need to develop and evaluate new antimicrobial agents. This scoping review offers an extensive and detailed synthesis of the potential role of selenium nanoparticles (SeNPs) in combating oral pathogens responsible for causing infectious diseases. A systematic search was conducted up until May 2022, encompassing the MEDLINE, Embase, Scopus, and Lilacs databases. We included studies focused on evaluating the antimicrobial efficacy of SeNPs on planktonic and biofilm forms and their side effects in in vitro studies. The selection process and data extraction were carried out by two researchers independently. A qualitative synthesis of the results was performed. A total of twenty-two articles were considered eligible for this scoping review. Most of the studies reported relevant antimicrobial efficacy against C. albicans, S. mutans, E. faecalis, and P. gingivalis, as well as effective antioxidant activity and limited toxicity. Further research is mandatory to critically assess the effectiveness of this alternative treatment in ex vivo and in vivo settings, with detailed information about SeNPs concentrations employed, their physicochemical properties, and the experimental conditions to provide enough evidence to address the construction and development of well-designed and safe protocols.
Full article
(This article belongs to the Special Issue Antimicrobial Agents Based on Nanomaterials)
►▼
Show Figures
Figure 1
Open AccessArticle
A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells
Pharmaceutics 2023, 15(9), 2252; https://doi.org/10.3390/pharmaceutics15092252 - 31 Aug 2023
Abstract
Mild photothermal therapy (PTT) shows great potential to treat cancers while avoiding unwanted damage to surrounding normal cells. However, the efficacy of mild PTT is normally moderate because of the low hyperthermia temperature and limited light penetration depth. Chemotherapy has unlimited penetration but
[...] Read more.
Mild photothermal therapy (PTT) shows great potential to treat cancers while avoiding unwanted damage to surrounding normal cells. However, the efficacy of mild PTT is normally moderate because of the low hyperthermia temperature and limited light penetration depth. Chemotherapy has unlimited penetration but often suffers from unsatisfactory efficacy in view of the occurrence of drug resistance, suboptimal drug delivery and release profile. As a result, the combinatory of chemotherapy and mild PTT would integrate their advantages and overcome the shortcomings. Herein, we synthesized an NIR-activatable and mild-temperature-sensitive nanoplatform (BDPII-gel@TSL) composed of temperature-sensitive liposomes (TSL), heat shock protein 90 (HSP90) inhibitor (geldanamycin) and photothermal agent (BDPII), for dual chemotherapy and mild PTT in cancer cells. BDPII, constructed with donor-acceptor moieties, acts as an excellent near-infrared (NIR) photothermal agent (PTA) with a high photothermal conversion efficiency (80.75%). BDPII-containing TSLs efficiently produce a mild hyperthermia effect (42 °C) under laser irradiation (808 nm, 0.5 W cm−2). Importantly, the phase transformation of TSL leads to burst release of geldanamycin from BDPII-gel@TSL, and this contributes to down-regulation of the overexpression of HSP90, ensuring efficient inhibition of cancer cell growth. This research provides a dual-sensitive synergistic therapeutic strategy for cancer cell treatment.
Full article
(This article belongs to the Special Issue Recent Progress in Reactive Oxygen Species-Related Therapy for Disease Treatment)
►▼
Show Figures
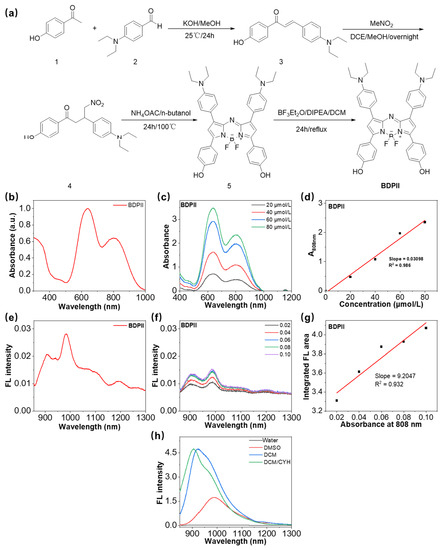
Figure 1
Open AccessReview
Cyclodextrins as Multi-Functional Ingredients in Dentistry
Pharmaceutics 2023, 15(9), 2251; https://doi.org/10.3390/pharmaceutics15092251 - 31 Aug 2023
Abstract
Cyclodextrins are present in a variety of oral hygiene compositions. The present work describes the role of cyclodextrins in several toothpastes and mouthwashes that are already available in the market, as well as their prospective use in other applications as investigated in studies
[...] Read more.
Cyclodextrins are present in a variety of oral hygiene compositions. The present work describes the role of cyclodextrins in several toothpastes and mouthwashes that are already available in the market, as well as their prospective use in other applications as investigated in studies in the literature. Moreover, cyclodextrins are under study for the development of materials used in various techniques of dental repair, such as fillings, cements and binders therein. Their role in each of the innovative materials is presented. Finally, the prospect of the use of cyclodextrin-based delivery systems for the oral cavity is introduced, with a focus on new cyclodextrin molecules with dual action as bone-targeting agents and osteogenic drugs, and on new cross-linked cyclodextrin particles with a high drug loading and sustained drug delivery profile for the treatment of diseases that require prolonged action, such as periodontitis. In conclusion, cyclodextrins are herein demonstrated to act as versatile and multi-action ingredients with a broad range of applications in dentistry.
Full article
(This article belongs to the Special Issue Biomaterials and Agents: Pharmaceutical and Biomedical Applications in Dental Research)
►▼
Show Figures
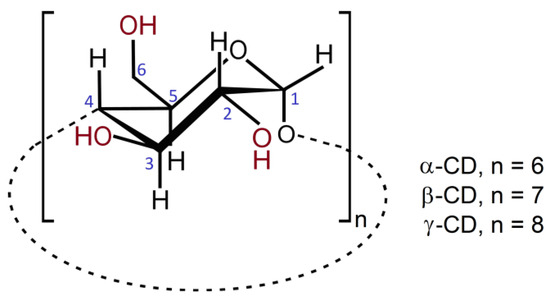
Figure 1
Open AccessArticle
Preclinical Investigation of a Lipoglycopeptide Dry Powder Inhalation Therapy for the Treatment of Pulmonary MRSA Infection
by
, , , , , , , , , , , and
Pharmaceutics 2023, 15(9), 2250; https://doi.org/10.3390/pharmaceutics15092250 - 31 Aug 2023
Abstract
The increased prevalence of pulmonary methicillin-resistant Staphylococcus aureus (MRSA) infection in patients living with cystic fibrosis (CF) is concerning due to a correlation with reduced life expectancy and lack of available treatment options. RV94 is a next generation lipoglycopeptide designed for pulmonary delivery
[...] Read more.
The increased prevalence of pulmonary methicillin-resistant Staphylococcus aureus (MRSA) infection in patients living with cystic fibrosis (CF) is concerning due to a correlation with reduced life expectancy and lack of available treatment options. RV94 is a next generation lipoglycopeptide designed for pulmonary delivery that preclinically demonstrated high potency against MRSA in planktonic and protected colonies and improved pulmonary clearance relative to same class molecules. Here, RV94 was formulated into a dry powder for inhalation (DPI) to investigate the localized treatment of pulmonary MRSA presented in a potentially more convenient dosage form. RV94 DPI was generated using a spray-drying process with 12.5 wt% trileucine and demonstrated aerosol characteristics (2.0 μm MMAD and 73% FPF) predictive of efficient pulmonary deposition. In vivo PK from a single dose of RV94 DPI delivered by inhalation to rats yielded lung levels (127 μg/g) much greater than the MRSA minimum inhibitory concentration (0.063 μg/mL), low systemic levels (0.1 μg/mL), and a lung t1/2 equal to 3.5 days. In a rat acute pulmonary MRSA model, a single dose of RV94 DPI delivered by inhalation either up to seven days prior to or 24 h after infection resulted in a statistically significant reduction in lung MRSA titer.
Full article
(This article belongs to the Special Issue Medical Aerosol Drug Delivery (Volume II))
►▼
Show Figures
Figure 1
Open AccessReview
Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application
Pharmaceutics 2023, 15(9), 2249; https://doi.org/10.3390/pharmaceutics15092249 - 30 Aug 2023
Abstract
►▼
Show Figures
Human acellular amniotic membrane (HAAM) has emerged as a promising tool in the field of regenerative medicine, particularly for wound healing and tissue regeneration. HAAM provides a natural biological scaffold with low immunogenicity and good anti-infective and anti-scarring results. Despite its potential, the
[...] Read more.
Human acellular amniotic membrane (HAAM) has emerged as a promising tool in the field of regenerative medicine, particularly for wound healing and tissue regeneration. HAAM provides a natural biological scaffold with low immunogenicity and good anti-infective and anti-scarring results. Despite its potential, the clinic application of HAAM faces challenges, particularly with respect to the preparation methods and its low mechanical strength. This review provides a comprehensive overview of HAAM, covering its preparation, sterilization, preclinical research, and clinical applications. This review also discusses promising decellularization and sterilization methods, such as Supercritical Carbon Dioxide (SC-CO2), and the need for further research into the regenerative mechanisms of HAAM. In addition, we discuss the potential of HAAM as a skin dressing and cell delivery system in preclinical research and clinical applications. Both the safety and effectiveness of HAAM have been validated by extensive research, which provides a robust foundation for its clinical application.
Full article
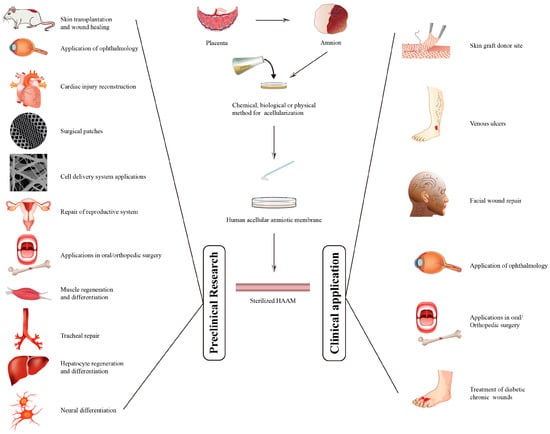
Figure 1
Open AccessArticle
Modified Curcuminoid-Rich Extract Liposomal CRE-SDInhibits Osteoclastogenesis via the Canonical NF-κB Signaling Pathway
by
, , , and
Pharmaceutics 2023, 15(9), 2248; https://doi.org/10.3390/pharmaceutics15092248 - 30 Aug 2023
Abstract
Curcuminoids, namely curcumin, demethoxycurcumin, and bisdemethoxycurcumin, are the major active compounds found in Curcuma longa L. (turmeric). Although their suppressive effects on bone resorption have been demonstrated, their pharmacokinetic disadvantages remain a concern. Herein, we utilized solid dispersion of a curcuminoid-rich extract (CRE),
[...] Read more.
Curcuminoids, namely curcumin, demethoxycurcumin, and bisdemethoxycurcumin, are the major active compounds found in Curcuma longa L. (turmeric). Although their suppressive effects on bone resorption have been demonstrated, their pharmacokinetic disadvantages remain a concern. Herein, we utilized solid dispersion of a curcuminoid-rich extract (CRE), comprising such curcuminoids, to prepare CRE-SD; subsequently, we performed liposome encapsulation of the CRE-SD to yield liposomal CRE-SD. In vitro release assessment revealed that a lower cumulative mass percentage of CRE-SD was released from liposomal CRE-SD than from CRE-SD samples. After culture of murine RANKL-stimulated RAW 264.7 macrophages, our in vitro examinations confirmed that liposomal CRE-SD may impede osteoclastogenesis by suppressing p65 and IκBα phosphorylation, together with nuclear translocation and transcriptional activity of phosphorylated p65. Blind docking simulations showed the high binding affinity between curcuminoids and the IκBα/p50/p65 protein complex, along with many intermolecular interactions, which corroborated our in vitro findings. Therefore, liposomal CRE-SD can inhibit osteoclastogenesis via the canonical NF-κB signaling pathway, suggesting its pharmacological potential for treating bone diseases with excessive osteoclastogenesis.
Full article
(This article belongs to the Special Issue Advanced Liposomes for Drug Delivery)
►▼
Show Figures
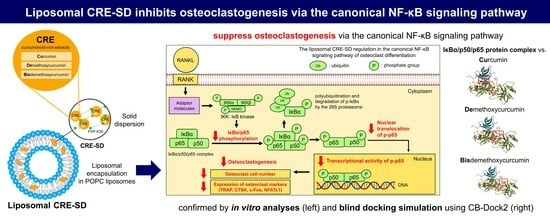
Graphical abstract
Open AccessReview
Hyaluronic Acid in Rheumatology
by
and
Pharmaceutics 2023, 15(9), 2247; https://doi.org/10.3390/pharmaceutics15092247 - 30 Aug 2023
Abstract
Hyaluronic acid (HA), also known as hyaluronan, is an anionic glycosaminoglycan widely distributed throughout various tissues of the human body. It stands out from other glycosaminoglycans as it lacks sulfation and can attain considerable size: the average human synovial HA molecule weighs about
[...] Read more.
Hyaluronic acid (HA), also known as hyaluronan, is an anionic glycosaminoglycan widely distributed throughout various tissues of the human body. It stands out from other glycosaminoglycans as it lacks sulfation and can attain considerable size: the average human synovial HA molecule weighs about 7 million Dalton (Da), equivalent to roughly 20,000 disaccharide monomers; although some sources report a lower range of 3–4 million Da. In recent years, HA has garnered significant attention in the field of rheumatology due to its involvement in joint lubrication, cartilage maintenance, and modulation of inflammatory and/or immune responses. This review aims to provide a comprehensive overview of HA’s involvement in rheumatology, covering its physiology, pharmacology, therapeutic applications, and potential future directions for enhancing patient outcomes. Nevertheless, the use of HA therapy in rheumatology remains controversial with conflicting evidence regarding its efficacy and safety. In conclusion, HA represents a promising therapeutic option to improve joint function and alleviate inflammation and pain.
Full article
(This article belongs to the Special Issue Hyaluronic Acid for Medical Applications)
►▼
Show Figures
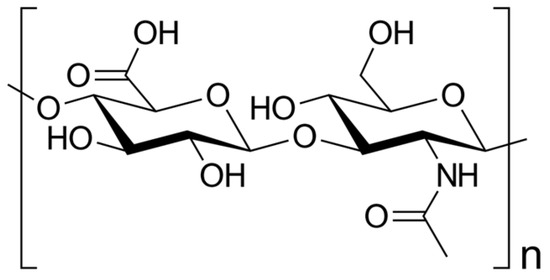
Figure 1

Journal Menu
► ▼ Journal Menu-
- Pharmaceutics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Antioxidants, BioChem, Biomolecules, Cells, IJMS, Nutrients, Pharmaceutics, Foods
Bioactive Compounds with Application Potentials in Nutraceuticals and Nutricosmetics: Focus on Mechanism of Action and Application Science
Topic Editors: Pujie Shi, Tiantian Lin, Lin Chen, Xin Yang, Caili Fu, Hyun-Gyun Yuk, Rong FanDeadline: 30 September 2023
Topic in
Cancers, Cells, Diagnostics, Future Pharmacology, Pharmaceutics
New Molecular Targets and Novel Strategies in Drug Development to Prevent Relapse in Acute Leukemia
Topic Editors: Oxana Dobrovinskaya, Ivan Delgado-EncisoDeadline: 31 October 2023
Topic in
Antioxidants, Biomolecules, Molecules, Pharmaceutics, Separations
Application of Analytical Chemistry in Exercise Physiology and Pharmacology
Topic Editors: Andrzej Pokrywka, Dorota KwiatkowskaDeadline: 15 November 2023
Topic in
Biomedicines, Cancers, Future Pharmacology, JCM, JNT, Pharmaceutics
Advances in Drug Delivery Systems Using Polymeric Nanocarriers
Topic Editors: Suhair Sunoqrot, Sara A. Abdel GaberDeadline: 30 November 2023

Conferences
Special Issues
Special Issue in
Pharmaceutics
Roles of Transporters and Receptors in Drug Delivery to the Brain in Health and Disease
Guest Editors: Gert Fricker, Elena PurisDeadline: 5 September 2023
Special Issue in
Pharmaceutics
New Properties of Supramolecular Complexes and Drug Nanoparticles
Guest Editors: Elena Uspenskaya, Anton SyroeshkinDeadline: 20 September 2023
Special Issue in
Pharmaceutics
Machine Learning for Dosage Forms Developments
Guest Editor: Svetlana IbrićDeadline: 30 September 2023
Special Issue in
Pharmaceutics
Ionic Liquids in Pharmaceutical and Biomedical Applications
Guest Editor: Roksana MarkiewiczDeadline: 20 October 2023
Topical Collections
Topical Collection in
Pharmaceutics
Feature Papers in Pharmaceutical Technology
Collection Editor: Thierry Vandamme
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Korea
Collection Editors: Hyo-Kyung Han, Beom-Jin Lee
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Estonia
Collection Editors: Karin Kogermann, Jana Lass
Topical Collection in
Pharmaceutics
Women in Pharmaceutics
Collection Editors: Donatella Paolino, Cinzia Anna Ventura